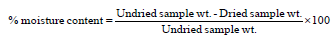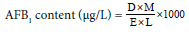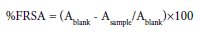Research Article
Assessment of Antifungal, Antiafl atoxigenic and Antioxidant Effi cacy of Ocimum Canum Sims Essential Oil and E-Citral as Phytopreservative against Aspergillus Flavus, a Toxigenic Food Spoiling Mould
Kumar A1* and Dubey NK2
1Department of Botany, Dr. Bhimrao Ambedkar Government Degree College, India
2Institute of Science, Banaras Hindu University, India
*Corresponding author: Kumar A, Department of Botany, Dr. Bhimrao Ambedkar Government Degree College, Maharajganj-273303, Uttar Pradesh, India, Mobile: +91-8400945878; E-mail: ashokkumarbhu@gmail.com
Copyright: © Kumar A, et al. 2020. Th is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 15/09/2020; Accepted: 19/10/2020; Published: 22/10/2020
Abstract
In the present study, stored wheat grains were found associated with spores of various storage moulds in which Aspergillus flavus exhibited the maximum relative density (33.48%). The chemical profi le of Ocimum Canum Essential oil (OcEO) showed 31 considerable peaks. E-citral (41.84%) was found as major component followed by Z-Citral (20.46%) and β-pinene (7.40%). OcEO and E-citral both exhibited broad spectrum fungitoxicity and their MIC against A. flavus LHPTA-07 was recorded at 0.6 and 0.4 mg/ml respectively while afl atoxin B1 production was completely checked at 0.4 and 0.3 mg/ml respectively. The gradual reduction in ergosterol content with increasing OcEO and E-citral concentration indicates plasma membrane as the possible target site of antifungal action. OcEO and E-citral also exhibited signifi cant antioxidant potential with IC50 value (16.72 and 15.44 μg/ml respectively) comparable to synthetic antioxidants (ascorbic acid, IC50 9.76 μg/ml) would be helpful to minimize lipid peroxidation. Fumigation of OcEO and E-citral signifi cantly reduced the population of A. flavus from wheat grains without adversely aff ecting seed germinability. Hence, OcEO and E-citral exhibited special virtues possessing antifungal, antiafl atoxigenic and antioxidant activity as well as no adverse eff ects on seed viability strengthening their safe exploitation as green preservative to enhance the shelf life of stored food commodities and other edible products.
Keywords
Aspergillus flavus; Ocimum Canum; Essential oil; Antifungal; Aflatoxin; Antioxidant
Introduction
Food commodities are susceptible to fungal and mycotoxin
contamination during postharvest storage and illness due to
consumption of these contaminated food products is a priority
concern to public health. In developing countries, postharvest
economic losses reach 25-40% or even more by fungal contamination
and the mycotoxins produced by them [1]. Among storage moulds,
diff erent species of Aspergillus potentially contaminate stored food
commodities and adversely aff ect their nutritive values [2]. Toxigenic
species of Aspergillus secrete afl atoxins in stored food commodities
and majority of population in developing countries are exposed
to afl atoxicoses [3]. Afl atoxin B1 is potent hepatocarcinogenic
and immunosuppressive, therefore, classifi ed as group-1 human
carcinogen by International Agency for Research on Cancer [4].
Various synthetic fungicides (Carbendazim, Mancozeb, Benomyl, Ceresan, Ziram etc.) have been used for a long time and have greatly contributed in management of such losses but due to their harmful health eff ects, resistance development in pathogens and residual toxicity [5]. Synthetic preservatives are also produce partially reduced oxides such as superoxide (O2−), hydrogen peroxide (H2O2) and also hydroxyl radicals (OH−) causing severe damage to biomolecules and also responsible for the stimulation of aflatoxin biosynthesis [6,7]. Hence, there is a need to develop some ecofriendly alternatives of these synthetic fungicides/preservatives.
Higher plants are great reservoir of various bioactive phytochemicals. Recently, plant based formulations are exploited as safe alternatives of synthetic preservatives and these green preservatives are Generally Recognized as Safe (GRAS) by U.S. FDA [8]. Various essential oils and their common bioactive components viz. terpenoids and phenolics have been used as antifungal [9], antimycotoxigenic [10], and pesticidal as well as potent free radical
scavengers [11,12]. Some essential oil based formulations viz. TALENT, EcoSMART and EcoPCOR have been used on large scale in food and agriculture industries [13].
In the present study, Ocimum Canum Essential oil (OcEO) and its major component E-citral has been investigated for their fungitoxicity against toxigenic isolate of A. flavus, a potent storage fungus causing postharvest loses of food commodities. In addition, OcEO and E-citral was also studied for their antioxidant activity as well as eff ect on seed viability for recommendation of OcEO as plant based food preservative.
Materials and Methods
Collection and preparation of wheat grains:
Stored wheat (Triticum aestivum L.) grains (variety-Malviya) were locally procured from retailers of Varanasi district of UttarPradesh, India. Th e grains were collected in sterilized low density poly ethylene bags to avoid further contamination. Th e grains were ground using a surface sterilized household blender. Th e powder was filtered through No. 50 mesh sieve and packed tightly in paper bags and stored at 5±2 ºC for further analysis [14].pH and moisture content determination:
For pH measurement, powdered wheat sample: distilled water
suspension (1:10; w/v) was prepared and stirred for 24 h in 200 ml
beaker. Th e pH of the suspension was measured using electronic pH
meter. To determine moisture content, weighed amount of powered
samples were dried at 100 ºC until their weights remained constant
and per cent moisture content was calculated as follows [15] -Mycological analysis of powdered wheat samples:
Storage moulds associated with wheat grains were assessed by
serial dilution method [16]. Th e isolated fungal species were identifi ed
on the basis of cultural and morphological characteristics [17,18].
Th e identifi ed fungal colonies were purifi ed and preserved on Potato Dextrose Agar (PDA) slant at 4±2 ºC. Th e per cent relative densities of
diff erent fungi on raw herbal drug samples were calculated following
[19].Detection of afl atoxigenic isolate of A. flavus:
Twenty isolates of A. flavus were randomly selected to determine their Afl atoxin B1 producing potential by Th in Layer Chromatography (TLC) following [20]. Fifty μl conidial suspension (≈106 conidia/ml) of selected A. flavus isolates were separately inoculated in 49.5 ml SMKY (Sucrose, 200 g; MgSO4.7H2O, 0.5 g; KNO3, 0.3 g; Yeast extract, 7.0 g; Distilled water, 1000 ml) broth medium in 150 ml Erlenmeyer fl ask and mixed properly followed by incubation at 27±2 °C for 10 days. Content of each fl ask was filtered aft er incubation and filtrate was extracted with chloroform (40 ml) in a separating funnel. The separated chloroform extract was dried on water bath at 60-70 °C.
Th e residue left aft er evaporation was re-dissolved in 1 ml chloroform and 50 μl of it was spotted on TLC plate (20×20 cm2 of silica gel). The plate was developed in toluene: isoamyl alcohol: methanol (90:32:2;
v/v/v) solvent system and intensity of AFB1 was observed under ultra violate fluorescence analysis cabinet at an excitation wavelength of 360 nm [21]. Th e fluorescent blue spots on TLC plate containing AFB1 were scraped in 5 ml cold methanol and centrifuged at 3000 rpm for 5 min. Absorbance of supernatant was recorded at 360 nm and AFB1 content was quantified following [22].Where, D = absorbance, M = molecular weight of AFB1 (312), E = molar extinction coeffi cient of AFB1 (21,800) and L = path length (1 cm cell was used)
Extraction of O. canum leaf essential oil (OcEO):
For essential oil extraction, leaves of O. canum were collected
from Banaras Hindu University campus, Varanasi. Th e plant was
identifi ed with the help of Flora of BHU Campus and its voucher
specimen (Lam/O-119/2019) was lodged in the herbarium of
department of botany [23], Banaras Hindu University. Leaves of
the plant were thoroughly washed with 1% Sodium hypochlorite
followed by distilled water. Th e volatile fraction (EO) of leaves was
extracted by Clevenger’s hydro-distillation apparatus. Th e extracted
OcEO was stored in dark clean glass vial aft er removing water traces
passing through anhydrous sodium sulphate and kept at 4-6 ºC [21].GC/GC-MS analysis of OcEO:
To determine the chemical composition, OcEO was analyzed through gas chromatography (Perkin Elmer Auto XL GC) equipped with a fl ame ionization detector. Th e GC conditions were as follows: column, EQUITY-5 (60 m x 0.32 mm x 0.25 μm); H2 was the carrier gas; column Head pressure 10 psi; oven temperature program isotherm 2 min. at 70 ºC, 3 ºC/min. gradient to 250 ºC, isotherm 10 min; injection temperature, 250 ºC; detector temperature 280 ºC. The GC-MS analysis was also performed using Perkin Elmer Turbomass GC-MS. Th e effl uent of the GC column was introduced directly into the source of MS. Spectra were obtained in the EI mode with 70ev ionization energy. Th e compounds were identifi ed by comparison of their relative retention times and the mass spectra with those of authentic reference compounds shown in the literature [24].Fungitoxic spectrum of OcEO and E-citral against some storage fungi:
Fungitoxic effi cacy of OcEO and its major component E-citral was also recorded against 12 storage moulds viz. Alternaria alternata, Aspergillus candidus, A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, Cladosporium cladosporioides, Curvularia lunata, F. oxysporum, Penicillium italicum and Trichoderma viride recovered from powdered wheat samples. Requisite amount of OcEO and E-citral dissolved separately in 0.5 ml of 5% tween-20 mixed with 9.5 ml PDA medium in diff erent presterilized Petri dishes to attain final concentrations i.e. 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5 and 2.0 mg/ml. Th e control sets were kept parallel to the treatment sets without OcEO. A positive control of bavistin (Carbendazim 50% WP), a prevalent synthetic fungicide was also kept parallel. A fungal disc (5 mm diameter) of each test fungus was inoculated separately and incubated at 27±2 °C for 7 days. Aft er incubation, Minimum Inhibitory Concentration (MIC) was recorded [25]. The concentrations on which OcEO and E-citral completely checked the visible fungal growth on PDA medium was considered as MIC.Antifungal and afl atoxin inhibitory effi cacy of OcEO and E-citral:
Fungitoxic and aflatoxin inhibitory efficacy of isolated OcEO and its major component E-citral was tested against the toxigenic isolate of A. flavus LHPTA-07 using SMKY (Sucrose, 200 g; MgSO2.7H2O, 0.5 g; KNO3, 0.3 g; Yeast extract, 7.0 g; Distilled water, 1000 ml) broth as nutrient medium following [20]. Requisite amount of the OcEO and E-citral dissolved separately in 0.5 ml of 5% tween-20 were pipetted aseptically to different presterilised Erlenmeyer fl asks (150 ml) containing 49.5 ml of SMKY broth to procure the final concentrations viz. 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 mg/ml. The control sets were kept parallel to the treatment sets without OcEO and E-citral. Th en, fl asks were inoculated aseptically with 50 μl spore suspension (≈106 spores ml-1) of toxigenic isolate of A. flavus LHPTA-07 prepared in 0.1 % Tween-80 and incubated at 27±2 °C for 10 days [26]. Th e content of each fl ask was filtered (Whatman no.
1) and mycelium was oven dried at 100 °C till their weight remained constant for biomass determination. Mycelial biomass of treatment and control sets was measured and per cent mycelial inhibition was calculated [21]. Th e filtrates of control and treated sets were extracted separately with 50 ml chloroform in a separating funnel to quantify the aflatoxin B1 production. Aflatoxin B1 production in each set was
estimated by aforementioned technique of [20].Eff ect of OcEO and E-citral on ergosterol content:
Eff ect of OcEO on ergosterol content in plasma membrane of A.
fl avus LHPTA-07 was assessed following [22]. Requisite amount of the
OcEO and E-citral was dissolved separately in 0.5 ml of 5% tween-20
were pipetted aseptically to diff erent presterilised Erlenmeyer fl asks
(150 ml) containing 49.5 ml of SMKY broth to procure the fi nal
concentrations from 0.1 to 0.6 mg/ml. Th e fl ask without OcEO and
E-citral was treated as control. Each fl ask was inoculated with 100 μl
spore suspension of A. flavus LHPTA-07 followed by incubation at
27±2 °C for 5 days. Recovered mycelia from treated and control sets
were subjected to extraction and quantifi cation of ergosterol.Free radical scavenging activity of OcEO and E-citral:
Th e antioxidant activity of the OcEO and E-citral was determined
by DPPH radical scavenging assay on TLC as well as its free radical
scavenging activity was measured through spectrophotometery
following [27]. Free radical scavenging activity of the OcEO and
E-citral was measured by recording the extent of bleaching of
the purple-coloured DPPH solution to yellow. Diff erent graded
concentrations (1.0 to 20.00 μg/ml) of the samples were added
separately to 4% DPPH solution in methanol (5 ml). Aft er a 30 min
of incubation at room temperature, the absorbance was taken against
a blank at 517 nm using spectrophotometer. Scavenging of DPPH
free radical with reduction in absorbance of the sample was taken
as a measure of their antioxidant activity following [28]. Ascorbic
acid was used as positive control. Per cent Free Radical Scavenging
Activity (FRSA) was calculated using the following formula –Where, Ablank is the absorbance of the control (without test compound), and Asample is the absorbance of the test compound.
Antifungal fumigant activity of OcEO and E-citral during storage:
To determine the antifungal effi cacy of OcEO during storage, 500
g of surface sterilized wheat grains were kept separately in four sets
in diff erent plastic containers having aerial volume 2.0 liters. Each set
(three containers) was inoculated with 1 ml spore suspension (≈106 spores/ml) of A. flavus LHPTA-07. Each set was fumigated with OcEO
(0.6 mg/ml), E-citral (0.4 mg/ml) and bavistin (0.8 mg/ml) separately
at their MIC against A. flavus one set run parallel as control without
any fumigation. All the containers were kept air tight and stored for
six months at room temperature i.e. 27±2 ºC [29,30]. Aft er storage,
mycological analysis of fumigated wheat grains was performed to
determine the effect of fumigation on number of isolates of A. flavus
following [16].Seed germination test:
Th e viability of fumigated wheat grains was tested by seed
germination test. Aft er six months of storage, 200 uninfested seeds
were taken from each treated group and soaked in distilled water for
3 h. Th ereaft er, seeds were aseptically transferred to Petri dishes of 15
cm diameter (20 seeds per Petri plate) containing moist fi lter paper
and incubated at 25±2 0C. Two hundred healthy and uninfested seeds
were taken from the market as control for comparison. Th e number
of seeds germinated within a week was recorded as viable [31].Statistical analysis:
All the experiments were accomplished in triplicate and data
were expressed as Mean±Standard error (SE) followed by one way
ANOVA (P < 0.05) and Tukey’s multiple range tests. Th e soft ware
SPSS (version 16.0) was used for statistical analysis of data.Results and Discussion
Mycofl ora analysis of powdered wheat grains:
Th e level of fungal contamination is mainly governed by pH and
moisture content. Th e collected wheat grains having appropriate pH (6.90±0.047) whereas their relatively higher moisture content
(17.64±0.347%) refl ects eff ect of conducive climatic conditions
of Indian subcontinent which enhance fungal and mycotoxin
contamination in food commodities during storage. During
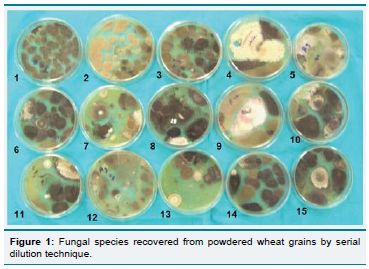
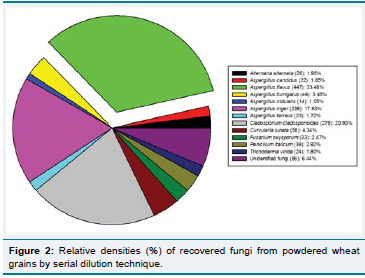
mycological analysis, total 1335 fungal isolates were recovered and
among them A (Figure 1). fl avus was dominated with highest relative
density (33.48%) followed by Cladosporium cladosporioides (20.92%)
and A. niger (17.83%) while A. nidulans exhibited the lowest (1.05%)
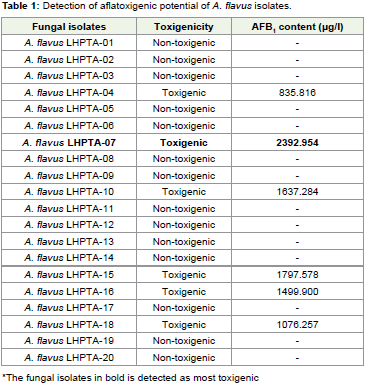
relative density (Figure 2). Th irty percent isolates of randomly selected
A. flavus were found toxigenic and A. flavus LHPTA-07 was selected
as test fungus due to its higher potential of afl toxin B1 production
(2392.654 μg/l) presented in Table 1. Th e Aspergilli usually exhibited
relatively higher density due to their strong capability to produce
some hydrolytic enzymes [32-34]. Fungal contamination also
degraded the nutritive values of food commodities and rendering
them unfi t for human consumption [2]. Some higher plant essential
oils can be exploited as substitute of synthetic fungitoxicant against
several moulds and their mycotoxin production [10,35]. An eff ort
was made to evaluate the antifungal effi cacy of OcEO and its possible
applicability in control of postharvest fungal deterioration of food
commodities during storage and also enhancing their shelf life.
Figure 2: Relative densities (%) of recovered fungi from powdered wheat
grains by serial dilution technique.
Extraction and chemical description of OcEO:
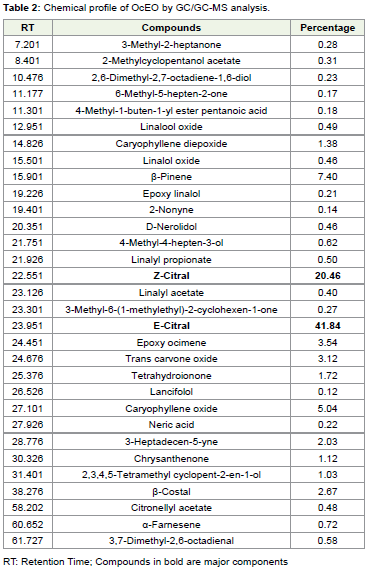
Th e OcEO was extracted through hydro-distillation is characterized with its pungent smell, yellow green colour and 0.96 % yield (w/w). Th e chemical profi le of EOs varies with age of the plant, season of collection, geographical area and soil characteristics [36,37]. Hence, OcEO was standardized to determine the chemical composition through GC-MS analysis before recommending for formulation. Th e GC-MS analysis of OcEO showed 31 considerable peaks in which E-Citral (41.84%) was found as major component
followed by Z-Citral (20.46%), β-Pinene (7.40%), Caryophyllene oxide (5.04%), Epoxy ocimene (3.54%), Trans Carvone oxide (3.12%), β-Costol (2.67%) and 3-Heptadecen-5-yne (2.03%). Rest other identifi ed components were found in trace amount (Table 2). Th e major component of OcEO is diff erent from earlier fi ndings where, camphor [38], linalool [39], eucalyptol [40], thymol [41], etc. were reported as major components.Antifungal and antiafl atoxigenic effi cacy of OcEO and E-citral:
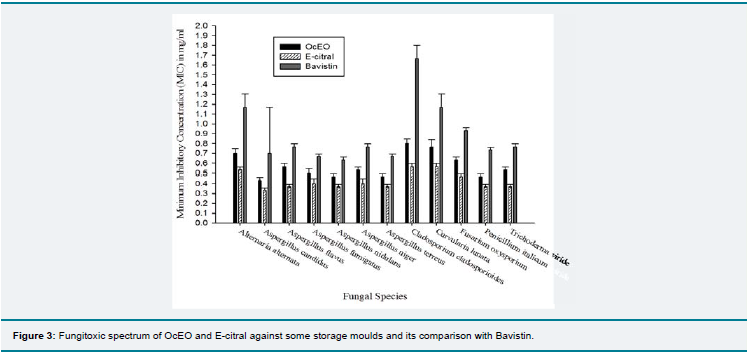
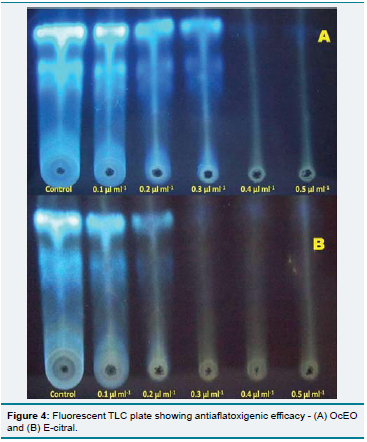
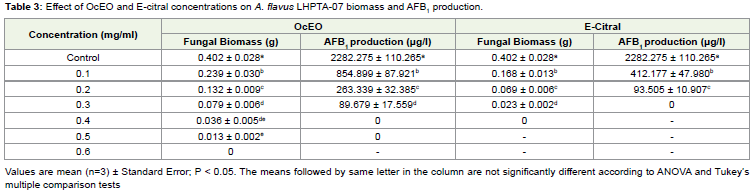
Th e OcEO and E-citral both exhibited remarkable broad fungitoxic spectrum at 1.0 mg/ml concentration against the 12 storage fungi isolated during mycological analysis and also showed superiority over synthetic fungicide bavistin (Figure 3). Th e broad spectrum fungitoxicity and superiority over bavistin would be suitable to provide complete protection from large number of fungal pathogens. Th e MIC of OcEO and E-citral against A. flavus LHPTA-07 was found to be 0.6 mg/ml and 0.4 mg/ml respectively whereas; OcEO and E-citral completely checked the AFB1 production at 0.4 and 0.3 mg/ml concentration respectively (Table 3 and Figure 4). A direct relationship has been observed between fungal biomass and aflatoxin production. The AFB1 production decreased with increasing concentrations of OcEO and E-citral. Fungal biomass
and AFB1 production exhibited a significant declining trend with increasing OcEO and E-citral concentrations. Therefore, to check AFB1 production, mycelial growth must be below the threshold limit so that aflatoxin could not be produced [42]. Th e OcEO completely checked the AFB1 production at lower concentration than earlier reported EOs viz. Cymbopogon flexuosus [31], Curcuma longa [43], Pimenta dioica [44], Coriandrum sativum [45], Artemisia nilagirica [46] etc. The antifungal and antiaflatoxigenic efficacy of OcEO may be due to bioactivity of different constituents or due to different metabolic pathways because; MIC and AFB1 inhibition was recorded at different concentrations. E-citral, a well known antifungal agent
[47,48] is major component (41.84%) of OcEO, may also played promising role in its fungitoxicity.Antioxidant activity of OcEO and E-citral:
Food commodities are also deteriorated by free radical mediated
oxidation of unsaturated lipids during storage [49]. Oxidative stress
stimulates A. flavus to produce more AFB1 during storage [7] which
results quantitative as well as qualitative losses to stored commodities
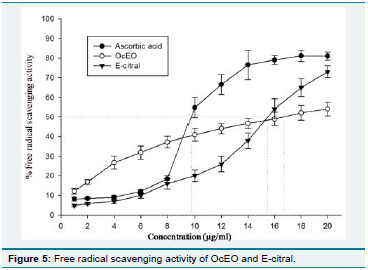
and reduces their shelf life. Th e OcEO and E-citral exhibited
signifi cant radical scavenging activity as their IC50 values (16.72 and
15.44 μg/ml respectively) in concentration dependent manner (Figure
5), which were found comparatively higher than ascorbic acid (9.76
μg/ml). Th e IC50 values of OcEO and E-citral were found greater than
EO of O. sanctum, O. gratissimum, O. basilicum etc. but quite lower
than some earlier reported EOs and also comparable to synthetic
antioxidants [39,50,51]. Th e presence of various phenolic compounds
and/or synergistic eff ect among compounds also play major role in
antioxidant activity of EOs [52,53]. Owing to free radical scavenging
activity, the OcEO and E-citral may serve as plant based antioxidants
in shelf life enhancement as well as protection from oxidative stress
by decelerating oxidative rancidity of lipids.
Figure 3: Fungitoxic spectrum of OcEO and E-citral against some storage moulds and its comparison with Bavistin.
Eff ect of OcEO and E-citral on fungal cell membrane:
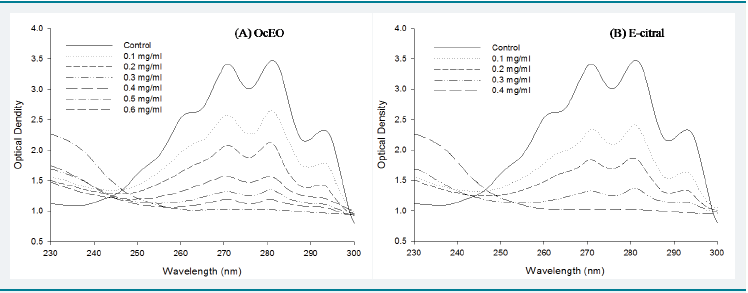
Ergosterol is specifi c sterol in fungal cell membrane providing
membrane integrity and fl exibility as well as stability of membrane
associated enzymes and signifi cant alteration of its biosynthesis
adversely aff ect fungal growth [54,55]. Ergosterol content of cell
membrane was found decreasing with increasing concentration of
OcEO and E-citral. Th e per cent inhibition of ergosterol contents by
OcEO treatment at 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 mg/ml concentration
was 33.86%, 55.02%, 77.14%, 86.43%, 92.86% and 100% respectively.
Whereas per cent ergosterol content inhibition by E-citral at 0.1, 0.2,
0.3 and 0.4 mg/ml concentration was found 43.57%, 65.71%, 85.71%
and 100% respectively (Figure 6). Th e decrease in ergosterol level
with increasing OcEO and E-citral concentration clearly denotes
that the bioactive components of OcEO targeted the cell membrane
and rendering them more permeable to leakage of ion and other cell
content [56].Eff ect of OcEO and E-citral on seed germinability:
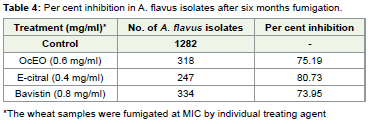
Th e OcEO and E-citral drastically reduces the A. flavus
population in fumigated wheat samples i.e. 75.19 and 80.73% at 0.6
mg/ml and 0.4 mg/ml concentrations respectively (Table 4). Th e
fumigant activity of OcEO and E-citral against A. flavus was high and
also comparable to vabistine where 73.95% reduction was recorded
(Table 4). Reduction of A. flavus isolates in fumigated wheat samples
of OcEO and its major constituent also showed their preservative
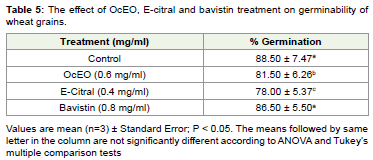
nature. OcEO and E-citral fumigated wheat grains showed 81.5% and
78.0% germination, which was also noteworthy (86.5%) to prevalent
synthetic fungicide vabistine strengthens their non-phytotoxic nature
even six months aft er application (Table 5).Th e plant O. canum is also used as phytomedicine in diff erent
diseases [57,58] exhibited its non-mammalian toxic nature. Th us,
non-phytotoxic and non-mammalian toxic nature of the OcEO
strengthens its possible exploitation as a safer plant based preservative
of food commodities during storage. Th e fi ndings may draw the
attention of food industries to conduct further experiments regarding
large scale exploitation of OcEO as botanical preservative for food
commodities during storage. Th e attraction of modern society in
‘green consumarism’ [59] desiring fewer synthetic ingredients in foods
and recommendation of herbal products as ‘Generally Recognized as
Safe’ (GRAS) in the developed countries may lead scientifi c interest in
OcEO as food preservative.
Table 3: Eff ect of OcEO and E-citral concentrations on A. flavus LHPTA-07 biomass and AFB1production.
Conclusion
Th e fi ndings of present study reveals that, OcEO exhibited
antifungal, antiafl atoxigenicity, broad fungitoxic spectrum, free radical
scavenging activity and non-phytotoxicity which strengthening its
exploitation as a substitute of synthetic preservatives for enhancing
the shelf life of stored food commodities and other edible products
during storage.
Acknowledgement
Th e authors are thankful to Head, Centre of Advanced Study in
Botany, Banaras Hindu University, Varanasi, India, for providing
laboratory facilities.