Research article
Symbiotic N Fixation: Plant-Microbe Interaction between Tepary Bean and Bradyrhizobium Strains
Michele D. Mohrmann1, Harbans L. Bhardwaj1*, Hua Shen2 and Regina Knight-Mason2
Agricultural Research Station, Virginia State University, PO Box 9061, Petersburg, VA 23806, USA
Department of Biology, Virginia State University, Petersburg, VA 23806, USA
Corresponding author: Harbans L. Bhardwaj, Agricultural Research Station, Virginia State University, PO Box9061, Petersburg, VA 23806, USA, E-mail:HBHARDWAJ@VSU.EDU
Citation:Mohrmann MD, Bhardwaj HL, Shen H, Knight-Mason R. Symbiotic N Fixation: Plant-Microbe Interaction between Tepary Bean and Bradyrhizobium Strains. J Plant Sci Res. 2017; 4(1): 167.
Copyright © Mohrmann MD, et al. 2017. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 4, Issue: 1
Submission: 23/03/2017; Accepted: 17/05/2017; Published: 29/05/2017
Abstract
Tepary bean is a drought-tolerant food legume that can provide nutritious food in many parts of the world especially arid areas [1]. Tepary bean being a legume can fix atmospheric N via Symbiotic N Fixation (SNF). However, detailed information about SNF by tepary bean is not available. We have previously reported that a commercial bradyrhizobial strain can nodulate tepary bean. We conducted two additional experiments to identify additional bradyrhizobial strains capable of nodulating tepary bean and to characterize the existence, if any, of specificity between strains and host plants. Results of the first experiment, when nodulation traits of black, tan, and white seeded lines inoculated with 15 bradyrhizobial strains were studied, indicated lack of any effects related to seed color, however, effects of bradyrhizobial strains were significant. Results of the second experiment, when nodulation traits of 31 tepary bean lines inoculated with 16 bradyrhizobial strains were studied, also indicated that bradyrhizobial strains differed significantly for their efficacy. In both experiments, there was lack of any specificity between bradyrhizobial strains and tepary bean lines. Our results indicated that in addition to Phaseolus Spec. #3 (Previously identified efficient nodulation strain, UMR-3255 and UMR-3043) are most efficient strains for SNF in tepary bean. We suggest that these strains could be useful in SNF in tepary bean. In addition, a composite of these strains could also be useful for field inoculations.
Introduction
Tepary bean is a desert legume native to the south western United States, Texas, New Mexico and Mexico. Tepary bean has served as a staple food for generations of prehistoric Native Americans [2]. Tepary bean’s dry seed is consumed due its rich protein varying from 17 to 27 percent and carbohydrate content averaging 59 percent [3]. Biochemical analysis of tepary bean indicates that tepary plants produce a high amount of soluble solids in the seed such as glucose and sucrose under sufficient or insufficient water supply [4]. Tepary bean plants flower within 27- 40 days after germination and ripen between 60-80 days [5]. Short duration wild tepary bean varieties mature within two months in tropical climates. However, its growth period may extend up to 120 days in cooler areas such as in costal Algeria.
Tepary bean is considered one of the most heat and droughttolerant crops in the world [3]. This legume has the ability to flourish during intense drought with low annual rainwater requirements ranging from 500 mm to 1700 mm [5]. The use of nitrogen fixed by the Tepary bean may eliminate and or reduce applying synthetic inorganic nitrogen fertilizer, thus reducing the possibility of water pollution caused by run-off into streams, lakes, rivers and tributaries. However, information related to Symbiotic N Fixation by Tepary bean is very limited. Duggar indicated that Tepary bean does not nodulate in Alabama (USA) soils [6]. We were the first to demonstrate that a commercial bradyrhizobial strain (Phaseolus Spec. #3) from EMD Crop BioScience, Inc. (Now Novozymes BioAg, Inc., Milwaukee, Wisconsin USA 53209) was able to produce functional nodules in tepary bean [5].
The current study was conducted during 2008 as a follow up toour earlier study to identify additional bradyrhizobial strains capableof nodulating tepary bean and to characterize the existence, if any, ofspecificity between strains and host plants [5].
Materials and Methods
We conducted two experiments in the greenhouse to study root nodulation in tepary bean. In both experiments, 30 cm tall plastic pots were filled with moistened planting material (1:1 sterilized vermiculite and filtered sand). Two seeds were planted in each pot approximately 3 cm deep. A pipette was used to draw the liquid inoculant out of a sterile container and then released from the pipette on the seed. This was done repeatedly for every pot using a new pipette. Care was taken to avoid cross contamination among rhizobial strains. After inoculation, the seeds were immediately covered with planting material and then watered liberally.
First experiment was planted on February 26, 2008, and harvested on April 15, 2008 using a Complete Randomized Block Design. In this experiment, nodulation traits of three tepary bean lines after inoculation with 15 bradyrhizobial strains were studied. The second experiment was planted on April 23, 2008 and harvested on June 8, 2008. In this experiment, nodulation traits of 31 tepary bean lines, inoculated with 16 bradyrhizobial strains, were studied. The sixteen bradyrhizobial strains consisted of 15 strains used in the first experiment plus a strain from Nitragin Company (Phaseolus Spec. #3, the strain that was used in previous study [5].
After about six weeks, plants in both experiments were harvested, their roots gently washed, and nodule size and nodule number scores were recorded each using the following scale: Nodule size scores (1=Small sized nodules similar in size to mustard seed; 2=Medium sized nodules and 3=Large sized nodules greater in size than soybean seed), Nodule number scores (1=Less than 5 nodules; 2=More than 10 nodules and 3=More than 20 nodules). Chlorophyll count of each plant was recorded prior to harvesting using a Minolta SPAD-502. A new variable named “Combined Score†was computed by summing values for nodule size scores, nodule number scores, and SPAD readings. All data were analyzed using SAS [7].
Result and Discussion
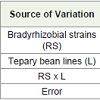
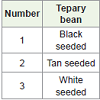
Significant variation existed among 15 bradyrhizobial strains in Experiment 1 (Table 1). However, variation among the three tepary bean varieties (VSU-Tepary-9-1 black-seeded, VSU-Tepary-9-2 tanseeded and VSU-Tepary-9-3 white-seeded) was not significant. There was a lack of specificity between bradyrhizobial strains and tepary bean lines as indicated by non-significance of interaction mean squares. Significant variation existed among 16 bradyrhizobial strains and 31 tepary bean lines in Experiment 2 (Table 2). Once again and similar to Experiment 1, there was a lack of specificity between bradyrhizobial strains and tepary bean lines as indicated by nonsignificance of interaction mean squares.
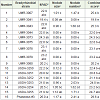
Table 1: Analysis of Variance (Mean squares) for nodulation traits from a greenhouse experiment when three tepary bean lines were inoculated with 15 bradyrhizobial strains.
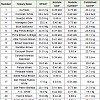
Table 2: Analysis of Variance (Mean squares) for nodulation traits from a greenhouse experiment when 31 tepary bean lines were inoculated with 16 bradyrhizobial strains.
Identification of Efficient Bradyrhizobial Strains forNodulation in Tepary Bean
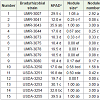
In Experiment 1, the maximum to minimum order of 15 bradyrhizobial strains for combined score was USDA-3255, UMR-3043, USDA-3254, USDA-3253, UMR-3007, USDA-3252, UMR- 3078, USDA-3250, UMR-3073, UMR-3080, UMR-3044, UMR-3079, UMR-3075, USDA-3251 and UMR-3041 when the combined score varied from 43.5 to 12.3 (Table 3). USDA-3255, strain with highest combined score of 43.5 also had the highest value for SPAD reading and nodule number score (39.5 and 3.0 respectively) whereas second highest value for nodule size score (1.0) which was statistically similar to strain 1 which had the highest numerical value of 1.08 for nodule size score. The strain with second highest combined score (UMR-3043) and the strain with the third highest combined score (USDA-3254) also had high values for the other three traits. These observations indicate that these three bradyrhizobial strains could be used for effective nodulation of tepary bean under field conditions. These results are expected to; in general, apply to most tepary bean lines. This expectation is based on the results where the 3 tepary bean lines didn’t differ for the traits under study (Table 4).
Table 3: Differences among 15 bradyrhizobial strains for nodulation traits averaged over 3 tepary bean lines from a greenhouse experiment.
Table 4: Seed color effects on nodulation traits of tepary bean lines after inoculation with 15 bradyrhizobial strains from a greenhouse experiment.
In Experiment 2, the maximum to minimum order of the 16 bradyrhizobial strains for the combined score was Phaseolus Spec. #3, UMR-3007, UMR-3041, USDA-3255, UMR-3073, USDA-3253, UMR-3079, USDA-3254, UMR-3078, USDA-3250, UMR-3075, UMR-3044, USDA-3252, USDA-3251, UMR-3080 and UMR- 3043 when the combined score varied from 29.6 to 19.6 (Table 5). Phaseolus Spec. #3 (From EMD Biosciences) also had the highest values for the three other traits under study. Bradyrhizobial strains UMR-3007 and UMR-3043, the strains with second and third highest combined scores also had high values for SPAD readings.
Table 5: Differences among 16 bradyrhizobial strains for nodulation traits averaged over 31 tepary bean lines from a greenhouse experiment.
Based on results of both experiments, it can be concluded that Phaseolus Spec. #3 (Best strain in Experiment 2), UMR-3255(Best strain in Experiment 1) and UMR-3043 (Best strain in both experiments) are the best bradyrhizobial strains for Symbiotic N Fixation in tepary bean. These strains could be used for effective nodulation of tepary bean under field conditions. These results should apply to most tepary bean lines. This expectation is based on the results where 9 out of 31 tepary bean lines had combined scores similar to each other (These tepary bean lines were associated with similar letters of Duncan’s Multiple Range Test) (Table 6).
Table 6: Nodulation traits of 31 tepary bean lines, averaged over 16 bradyrhizobial strains, from a greenhouse experiment.
On the other hand, a composite of these strains could also be useful for field inoculations given that Phaseolus Spec. #3 is a composite of three bradyrhizobial strains.
The experiments conducted under the current study indicate that bradyrhizobial strains are available to efficiently nodulate tepary bean contrary to the observations made by Dugger in 1934 that tepary bean did not nodulate in Alabama fields for use as SNF [6]. The results also indicate that nodulation and subsequent use of SNF capabilities of tepary bean are possible. The current study has identified that bradyrhizobial strains Phaseolus Spec. #3, UMR-3255, and UMR- 3043 are efficient nodulators of tepary bean.
Identifying the most efficient nodulators plays a vital role in determining the selection of tepary bean plants and bradyrhizobial strains that are most beneficial when utilizing tepary bean plants for SNF capabilities, increasing soil fertility and as an annual summer legume cover crop. This study also indicated that tepary bean plants with higher SPAD readings (an indication of higher relative greenness) after inoculation with the above bradyrhizobial strains produced increased nodule numbers. Nodule number and nodule size, two important traits for SNF, had a highly positive correlation (r=0.90).
The significance of these results is that the tepary bean is a summerannual legume crop with high potential for use as a summer legume cover crop without having detrimental effects on soil moisture. Tepary bean has low water requirements for growth and development, and also has the capability of high heat or drought tolerance resistance than common beans including navy, red kidney, and pinto beans.
The current study is a second report related to nodulation and SNF in tepary bean. In the first study, conducted at Virginia State University, it was indicated that tepary bean can be nodulated by inoculation with Phaseolus Spec. #3, a bradyrhizobial strain from Novozymes BioAg, Inc., Milwaukee, Wisconsin, USA 53209 [5]. Additionally, the first study indicated the feasibility of effectively nodulating the tepary bean germplasm and differentiated among the most and least efficient nodulators of tepary bean. The current study considerably expands the knowledge base related to SNF and tepary bean. It is suggested that additional studies with a wider array of tepary bean lines and bradyrhizobial germplasm be conducted. The tepary bean germplasm at Virginia State University consists of approximately 200 lines that potentially increase the foundation of tepary bean research and provide a complete assessment of bradyrhizobial strain interactions with selected tepary bean germplasm. However, additional efforts are needed to collect and characterize bradyrhizobial germplasm for tepary bean nodulation from tepary bean’s native areas of southwestern USA.
Conclusion
This study demonstrated that several bradyrhizobial strains can inoculate tepary bean and a lack of specificity between bradyrhizobial strains and tepary bean genotypes. It is suggested that additional studies with a multitude of tepary bean lines and bradyrhizobial germplasm be conducted to further characterize Symbiotic N Fixation in tepary bean for use in sustainable crop production in arid areas.
Acknowledgement
Our sincerest thanks are due Dr. Peter Graham (University of Minnesota) for providing the 15 bradyrhizobial strains for investigation. We are also thankful to Mr. John Kosanke (Novozymes BioAg, Inc., Milwaukee, Wisconsin, USA 53209), for providing the Phaseolus Spec. #3 bradyrhizobial strain; and Dr. Peter van Berkum (USDA-ARS) for his advice for this study and for serving as a cooperator for the grant proposal “Biological N Fixation and Seed Composition Traits of Tepary Bean†which was funded by US Department of Agriculture, National Institute Food and Agriculture. Funds from this grant were used to support Michele Mohrmann during her MS Degree and conduct of various experiments.
References
- Brink M, Belay G (2006) Plant resources of tropical Africa 1 Cereals and pulses. Prota foundations, Blackhuys publishers, CTA, Wageningen, Netherlands. pp.11-298.
- Cherrington M (2006) Two countries, One people. Land Res Environ Mongolia.
- Nabhan GP, Felger RS (1978) Teparies in southwestern North America: A biogeographical and ethnohistorical study of Phaseolus acutifolius. Econ Bot 32: 3-19.
- Coyne DP, Serrano JL (1963) Diurnal variations of soluble solids, carbohydrates and respiration rate of drought tolerant and susceptible bean species and varieties. Proc Am Soc Hort Sci 83: 453-460.
- Mapp CD, Knight-Mason R, Bhardwaj HL (2016) Symbiotic N Fixation: Nodulation of tepary bean (Phaseolus acutifolius A. Gray). J Plant Sci Res 3: 1-5.
- Duggar JF (1935) The nodulation and other adaptations of certain summer legumes. J Am Soc Agron.
- SAS (2014) SAS system for window, SAS institute Inc. Cary, NC.