Research Article
Phytochemical Analysis and AntimicrobialActivity of Lawsonia inermis (Henna)
Nirmala Babu Rao1* SitaKumari O2 and Rajesh Goud Gajula3
1Department of Botany, Osmania University, Hyderabad, Telangana, India
2Department of Botany, R.B.V.R.R. Women's College, Hyderabad, Telangana, India
3Department of Biotechnology, Primer Biotech, Hyderabad, India
Corresponding author: Nirmala Babu Rao, Department of Botany, Osmania University, Hyderabad, Koti, Hyderabad, Telangana, India; E-mail: rajesh4gajula@gmail.com
Citation: Rao NB, SitaKumari O, Rajesh Goud G. Phytochemical Analysis and Antimicrobial Activity of Lawsonia inermis (Henna). J Plant Sci Res.02 2016;3(2): 158.
Copyright © Rao NB, et al., 2016. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 3, Issue: 2
Submission: 25/07/2016; Accepted: 25/08/2016; Published: 30/08/2016
Abstract
Lawsonia inermis L. is a well-known plant usually grown in houses. It has various traditional medicinal and pharmacological values. It is much branched shrub or small tree cultivated for its leaves, beyond this stem, bark, roots, flowers and seeds also have great medicinal use. The aim of this experiment is to examine phytochemical and antimicrobial activities of Lawsonia inermis L. In recent studies, it is reported to have analgesic, hypoglycaemic, hepatoprotective, immunostimulant, antiinflammatory, antibacterial, antimicrobial, antifungal, antiviral, antiparasitic, antitrypoanosomal, antidermatophytic, antioxidant,antifertility, tuberculostatic, anticancer properties. The antimicrobial analysis is carried out by Agar well diffusion method. Clinical isolates Staphyloccus aureus, Streptocoocus sp, Pseudomonas aeuginosa, Candida albicans, Fusarium oxysporum and Aspergillus niger. Among those cultures Lawsonia leaves extract were able to inhibit the growth pattern of A. niger and F. oxysporun. Streptococcus sp and S. aureus were also inhibited by the extracts. The experiment which we conducted the phytochemical analysis with the crude extract of Lawsonia inermis L (Henna plant) gives various residues or components such as Tanins, flavonoids, alkaloids, terpenoids, Saponins, cardiac glycosides, glycosides, reducing sugars, phlobatanins, steroids, phenolic, aminoacids, proteins, quinones. There is absence of anthraquinones in Lawsonia by extracting with distilled water, ethanol, methanol and acetone. By following all these studies, lawsonia now considered as most valuable resource for unique natural products and also in the development of pharmacological products.
Introduction
Herbal medicines are in great in demand in the developed as wellas developing countries for primary healthcare because of their widebiological and medicinal activities, higher safety margins and lessercosts [1,2]. Medicinal plants are a part of human society to combat diseases from the drawn civilization [3]. In modern days drugs have their origin in their traditional plant medicine [4]. The therapeuticeffects of indigenous plants for several disorders have been describedby practitioners of traditional herbal medicines [5]. Natural products are the major sources of synthetic and traditional herbal medicine and are still the primary health care system [6]. This plant is usually called as Henna, mehendi plant and abundantly available in tropical and subtropical areas. Ancient history of India describes its diverse uses and also plays appreciable role in ayurvedic or natural herbal medicines [7]. Isolation of microbial agents less susceptible to regular antibiotics and revovery of resistant isolates during antibacterial therapy is increasing throughout the world [8].
Lawsonia inermis Linn
Genus Lawsonia consists of one species, L. inermis (Henna, mehendi, shudi, Madurang, Mendi, Manghati, Madayantika and goranti) [9,10]. It has synonyms as alba and spinosa belonging to family Lythraceae. It is biennial dicotyledons herbaceous herb. The origin of henna plant is North Africa and south west Asia. It is grown as an ornamental and dye plant.
It is much branched glabrous shrub or small tree 2 to 6m in height. Leaves are small, opposite in arrangement along the branches, sub-sessile, about 1.5 to 5 cm long, 0.5 to 2 cm wide, greenish brownto dull green, elliptic to broadly lanceolate with entire margin, petioleshort and glabrous and acute or obtuse apex with tapering base. Young branches are green in colour and turn red with age. Bark is greyish brown, unarmed when young but branches of older trees are spine tipped. Inflorescence is a large pyramid shaped cyme. Flowers are small about 1cm across, fragrant, white or rose coloured with four crumbled petals. Calyx is 0.2 cm tube and 0.3 cm spread lobes. Fruit is a small brown coloured round capsule. Fruit opens irregularly and splits into four sections at maturity and is many seeded. Seeds are about 3mm across, numerous, smooth, pyramidal, hard, thick seed coat with brownish coloration [10,11,12].
Henna plant has several ethnobotanical uses. It is widely used as medicinally and cosmetically. Henna leaves consist of 0.5-1.5% lawsone which is responsible for orange red dye color. Ithas an orange red dye and leaf paste or powder is used for decorating hands, nails and feet. It can also be used as a hair dye. Henna leaves are used to cure jaundice, skin diseases, veneral diseases, smallpox, spermatorrhoea. Henna leaves, flowers, seeds, stem bark, roots are used agent to treat ailments as rheumatoid arthritis, headache, ulcers, diahorrea, leprosy, fever, eucorrhea, diabetes, cardiac disease, hepatoprotective and coloring [13,14].
Material and Method
Study area
Plant materials were obtained from the botanical garden at Department of Botany in Osmania University by Prof. Nirmala baburao. Fresh leaves were collected and washed under running water to remove all the dust and dries in the oven at a temperature of 60°C. The dried leaves were ground into powder.
Extract Preparation
10gm of leaf powder was taken and added 50ml of ethyl alcohol stirred it constantly for 30 minutes and the solution was kept in room temperature for 24hrs and then filtered with muslin cloth to removefine particles. The filtered solution is again filtered with whatmannfilter paper No.3 and then stored at 4°C in a freezer for further use.
Preparation of Solutions
a) Fehling's solution: Take a beaker and add equal mixture of equal volume of copper sulphate, sodium potassium tartarateand sodium hydroxide.
b) Wagner's Reagent: Add 2gm of Iodine and 6gm of potassium iodide and mix well in 100ml of water.,
Chemicals such as wagner’s reagent, chloroform, 2% H2SO4, concentrated sulphuric acid,10%lead acetate, Benedicts reagent,0.1% ferric chloride, Fehlings solution, dilute NaOH, 2% HCL, 10% ammonia,10% HCL, distilled water, Ethyl alcohol are provided by themanagement of the college.
Phytochemical Screening
Chemical tests are carried out on the ethyl alcohol crude extract oflawsonia plant using standard procedure to identify the constituents.
• Alkaloids: To identify the presence of alkaloids, 2ml of extract and 2ml of wagner’s reagent is added. Brownish precipitate indicates the presence of alkaloids.
• Cardiac glycosides: To know the presence of cardiac glycosides, 2ml of extract is dissolved with 2ml of chloroform following by adding concentrated sulphuric acid carefully to form a layer. Deep reddish brown colour at the interface of steroid ring indicates the presence of cardiac glycosides.
• Flavonoids: To indicate the presence of flavonoids, 2ml extract is treated with 2ml of 10% lead acetate. Yellowish green colour indicates the presence of flavonoids.
• Saponins: To test the presence of saponins, 2ml of extract is dissolved with 2ml of benedicts reagent. Blue black pptindicates the presence of saponins.
• Tanins: To identify the presence of tanins, 2ml of extract with 0.1% ferric chloride.Brownish green indicates the presence of tanins.
• Terpenoids: ( Salkowski test) To know the presence of terpenoids, 2ml of extract is dissolved with 2ml of chloroform and concentrated sulphuric acid carefully to form a layer. A reddish brown colour indicates the presence of terpenoids.
• Anthraquinones: 1ml of extract is boiled with 10% HCL for few minutes in a water bath. It is filtered and allowed to cool. Equal volume of CHCL3 is added to the filterate few drops of 10% Ammonia is added to the mixture and heat. Formation of rose pink colour indicates the presence of anthraquinones.
• Reducing sugars: The extract was shaken with distilled water and filtered. The filterate was boiled with Fehlings solution A and B for few minutes an orange red ppt indicates thepresence of reducing sugars.
• Glycosides: To test the presence of glycosides, the extract was hydrolysed with HCL solution and neutralized with NaOH solution. A few drops of Fehlings solution A and B are added, red ppt indicates the presence og glycosides.
• Phlobatanins: To identify the presence of phlobatanins, the extract is dissolved in distilled water and filtered. The filterate is boiled with 2% HCL solution. Red ppt shows the presenceof Phlobatanins.
Antimicrobial activity
Specimen cultures: To conduct antimicrobial activity of Lawsonia inermis L (Henna plant), leaves were collected and washedunder running water to remove dust particles and air dried. The leaveswere grounded into fine powder. The powder was extracted with ethylalcohol and rota vaporized at 40-50°C for 3-4hrs to get crude extract.This extract was further used to evaluate antimicrobial activity.
Bacterial pathogens included in this study were Staphylococcusaureus, Pseudomonas aeruginosa, Streptococcus sp. The fungal pathogens used for testing are Aspergillus niger, Fusarium oxysporum and Candida albicans.
Inoculum Preparation
Bacterial pathogens were grown on nutrient agar and maintainedon slants while the fungi were grown on potato dextrose agar.
Measurement of zone of inhibition in diameter
For both fungi and bacteria, agar well diffusion method was used. 5ml of each concentration of the extracts was measured and the agar which is melted is poured in sterile petri dishes and left to solidify. The plates were inoculated with each of the various fungi and bacteria. Ditches are made with rod on agar plates. So the crude extract obtained with chloroform or ethyl alcohol can be inoculated. Standards are maintained for antibacterial and antifungal activities. The plates are being incubated for a week after inoculation so that culture can grow. The inoculated plates were observed on daily and readings were taken in diameter with the help of scale. The method which is used for measurement is linear measurement method.
Ampicillin (anti-bacterial), Itraconazole or Griseofulvin(antifungal) as the standard drug at a concentration of 200μg/ml. LBAgar was used as the culture media and potassium dextrose was usedas antifungal activity.
Results and Discussion
By observing the results of phytochemical analysis, thus weconclude that Lawsonia consists of high medicinal and therapeutic sources. A drug can be developed by undertaking the modern drugs isolated from henna plant. It plays a major role in pharmacological activity spectrum. In modern days people looking for nontoxic medication so it can used as traditional medicine and without any toxicity and side effects. Lawsonia is inhibiting many microorganisms and it can be useful wound healing, antiviral, antibacterial, antifungal, anti-inflammatory etc. By considering these results we can carried out for further experiment to get more medicinal and industrial products.
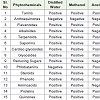
Table 1indicates the production of chemical components from Lawsonia leaf extract. It contains carbohydrates, proteins, flavonoids, tannins, phenolic compouns, alkaloids, terpenoids, quinones, coumarins, xanthones, fatty acids. Anthraquinones are absent inLawsonia by extraction with distilled water, methanol, acetone, ethanol.Due to the presence of Pharmalogical chemical components it can be used as analgesic, hypoglycemic, hepatoprotective, immunostimulant, anti-inflammatory, antibacterial, antimicrobial, antifungal, antiviral,antiparasitic, antitrypanosaomal, antidermatophytic, antioxidant,antifertility, tuberculostic and anticancer properties. It clearly showsthat Lawsonia is considered as valuable source for traditional andindustrial products.
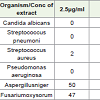
Table 2 showing the results of antimicrobial activity of Lawsonia inermis leaves crude extract. The antimicrobial activity is due to the presence of compound Lawsone. For S. aureus it shows inhibition in all the concentrations. In P. aeruginosa only mild antibacterial activity is observed. In streptococcus, less antibacterial activity has recorded it shows high inhibition at high concentrations.
At Candida albicans there is no activity. For the fungi, A. niger and Fusarium there is mild inhibition. The inhibition rate will beincreased by increasing the concentrations. This antimicrobialexperiment reveals that Lawsonia leaves are capable of inhibiting thebacterial and fungal pathogens.
References
- Cragg GM, Newman DJ, Sander KM (1997) Natural products in drug discovery and development. J Nat Prod 60: 52-60.
- Padma TV (2005) India Ayurveda Nature 436: 486.
- Bandyopadhyay U, Biswas K, Chattopadhyay I, Banerjee RK (2002) Biological activities and medicinal properties of neem (Azadirachtaindica). Currnt Sci 82: 1336-1345.
- Blanks T, Brown S, Cosgrave B, Woody J, Bentley V, O’ Sullivan N, Graydon N (1998) The Body shop Book of Wellbeing mind, body, and soul. Ebury Press London, pp.173-192.
- Natarajan V. Venugopal PV, Menon T (2003) Effect of Azadarichta indica (neem) on the growth pattern of dermatophtyes. Indian J Med Microbiol 21: 98-101.
- Singh A, Singh DK (2001) Molluscicidal activity of Lawsoniainermis and its binary and tertiary combinations with other plant derived molluscicides. Indian J Exp Biol 39: 263-268.
- Lavhate MS, Mishra SH (2007) A review: nutritional and therapeutic potential of Ailanthus excelsa. Pharmacog Rev 1:105-113.
- Bonjar SGH (2004) Screening for Antibacterial properties of some Iranian plants against two strains of Eschericia coli. Asian J Plant Sci 3: 310-314.
- Gupta AK (2003) Quality standards of Indian medicinal plants. Indian council of medicinal research 1: 123-129.
- Sastri BN (1962) The Wealth of India: Raw Materials. Edn 6, Vol. (L-M), CSIR, New Delhi 47-50P.
- Chauhan MG, Pillai APG (2007) Microscopic profile of powdered drug used in Indian system of medicine, Edn 1, Vol. 2, Gujarat Ayurved University, Jamnagar, Gujarat 84-85p.
- Vasudevan TN, Laddha KS (2003) Herbal drug microscopy, Edn 1, Yucca publishing house, Dombivli, pp. 68-69.
- Chetty KM (1956) Flowering plants of Chittoor, Edn 1, Andhra Pradesh, 2008, pp. 132. 11. Chopra RN, Nayer SL, Chopra IC. Glossary of India Medicinal Plants, CSIR Publications, New Delhi, 151p.
- Reddy KR (1988) Folk medicine from Chittoor district Andhra Pradesh, India used in the treatment of jaundice. International Journal of Crude Drug Research 26: 137-140.