Review Article
Some Conspicuous Traits in Domesticated Rice Oryza Sativa, Due To Recessive Alleles
Anjan Barman1*, Lingaraj Sahoo2 and Suvendra Kumar Ray3
1,3Department of Molecular Biology and Biotechnology, Tezpur University, Napaam, Tezpur- 784028, Assam, India
2Department of Biosciences and Bioengineering, Indian Institute of Technology, Guwahati, Assam 781 039, India
Corresponding author: Anjan Barman, Department of Molecular Biology and Biotechnology, Tezpur University, Napaam, Tezpur- 784028, Assam, India; E-mail: abarman@tezu.ernet.in
Citation: Barman A, Sahoo L, Ray SK. Some Conspicuous Traits in Domesticated Rice Oryza Sativa, Due To Recessive Alleles. J Plant Sci Res. 2016;3(1): 143.
Copyright © 2016 Anjan Barman, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 3, Issue: 1
07/01/2016; Accepted: 17/02/2016; Published: 23/02/2016
Abstract
Evolutionarily organisms are bestowed with salient morphological features which are considered to be an outcome of activity of functional gene products. However, there are several important features present in organisms owing to recessive alleles in specific gene loci. Interestingly, prevalence of such features due to recessive alleles of gene(s) raises intriguing questions in regard to evolution of the functional alleles in organisms! To have some understanding ofthis unique phenomenon, we examined four frequently confronted recessive traits in rice plant (Oryza sativa) such as (i) fragrance (ii) semi-dwarf height (iii) resistance to pathogens and (iv) non-seed shattering feature and discussed in brief the genetics, biochemistry and evolutionary significance of these traits in rice. These important traits in rice are due to mutations in genes such as BADH2, sd1, Xa13 and seed-shattering loci respectively, that otherwise encode essential functional attributes in plant systems. Genes with paralogs possibly attribute to recessive traits through their differential expression patterns. New facts are evolving on role of epigenetic mutations conferring heritable changes in gene expression that are not due to changes in the underlying gene sequence.
Keywords: Green revolution rice; Recessive trait; Resistance to plant-pathogen; Rice fragrance
Introduction
Generally, all observable features in an organism are perceivedas functional gene attributes. However, this is not the case always.Interestingly, there are certain features in organisms that areconsequences of non-functional genes rather than functional ones. Itis difficult to elucidate how non-functional gene contributes toward afeature in an organism. However, there are several examples in naturewhere non-functional alleles confer distinct traits to organisms. Forinstance, several features like fruit shape and size, stature of plants,fragrance of seed etc. established in commonly cultivated plantsare due to occurrence of non-functional or recessive alleles in theirgenomes [1-6]. It is noteworthy that prevalence of these kinds offeatures also has contributed immensely towards their domesticationprocesses [1,7]. The features due to non-functional or recessive alleles follow quantitative mode of inheritance (Figure 1a) and generallydo not comply with the dominant mode of inheritance (Figure 1b).Due to recessive or quantitative pattern of inheritance in these traits,identifying them without specific selection procedures is not easy.
Figure 1: (a): A cross between a tall plant (with 7nos leaves) and a dwarf plant (with 3nos leaves) giving rise to F1 generation plant bearing phenotypic features intermediate between both the parent plant i.e. intermediate height and leaf number(5nos). This inheritance pattern follows quantitative modeand differ from typical Mendelian inheritance trend. Generally, polymorphisms at specific QTLs occurring in the genome account for such phenotypic expressions. (b) A cross between a tall plant (with 7nos leaves) and a dwarf plant (with 3nos leaves) giving rise to F1 generation plant bearing phenotypic features of the tall parent plant (with 7nos leaves) which is the dominant of the two parents. This follows typical Mendelian inheritance pattern.
In domesticated rice (O. sativa), several important traits areknown to be due to recessive or non-functional alleles at specificQTLs [3-14]. To be precise, few conspicuous features of O. sativalike seed fragrance, semi-dwarf stature, resistance to pathogen etc.have established its significance in agronomy. These qualities areresult of mutated or recessive alleles at specific QTLs, coding for nonfunctionalprotein/enzyme. As a matter of fact, presence of recessivealleles in them doesn’t hinder other important characters. Here, wesummarize four important conspicuous traits in O. sativa that allowthem to sustain even in the absence of functional alleles without reduction in their yield.
Rice fragrance
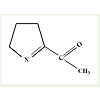
Fragrance is considered an important criterion for determiningquality of a particular cereal and also it is used to comprehend acereal’s endemic identity [2,4]. Basmati rice of India and Jasminerice of Thailand are important because of their distinctive fragrances.Research in this aspect has revealed that fragrance trait in rice is dueto a single gene locus on chromosome 8 called fgr [8,9]. A mutationin wild type BADH2 gene of this locus that code for a betainealdehyde dehydrogenase enzyme results in recessive allele badh2.1.It is believed that the truncated protein product of this recessiveallele is unable to catabolize the oxidation of ϒ-aminobutyraldehydewhich is normally catabolized by intact BADH2 protein [15,16].This defective pathway leads to accumulation of a precursor of thecompound 2-acetyl-1-pyrroline (2AP; Figure 2) and thus enhancedsynthesis of 2AP. The characteristic fragrance in rice is due to thepresence of this compound i.e. 2AP [17,18]. Though there is anotherisoform of betaine aldehyde dehydrogenase gene in rice (denoted asBADH-1 located in chromosome 4), it plays no role in rice fragrance[19]. Furthermore, fragrance due to 2AP resembles that of pandan(Pandanus amaryllifolius L.) and is exhaled by several other plantssuch as cucumber (Cucumis sativus L.), aromatic soybean (Glycinemax (L.) Merr.), aromatic sorghum (Sorghum bicolor (L.) Moench)etc. [6]. It appears that BADH-2 gene and metabolite 2AP has wideincidence amongst different generas of plants despite their differencesat phylogenetic level.
Figure 2: (a): Chemical structure of 2-acetyl-1-pyrroline, redrawn after Buttery et al. [45]. A mutation in wild type BADH2 gene of a single locus on chromosome 8 of rice that code for a truncated protein is unable to catabolize the oxidation of Ï’-aminobutyraldehyde [15,16]. This defect in the pathway lead to accumulation of a precursor of this compound and thus emanating enhanced synthesis of 2AP. The characteristic fragrance in rice is due to the presence of this compound i.e. 2AP [17,18].
Green revolution rice
Green revolution rice refers to semi-dwarf rice variety IR8 thatwas introduced in 1960s as a measure to increased global fooddemand.The characteristic short stature of the rice variety protectsthem from rain and wind, allowing efficient use of nitrogen fertilizersin them and hence contributes to its yield [3,20,21]. Semi-dwarftrait in IR8 is due to mutation in a gene called sd1 (semi dwarf).Basically sd1 comprises of two independent alleles located in thechromosome 1 of rice [22]. Wild type sd1 encodes an oxidase enzymethat metabolizes bio-synthesis of the phyto-hormone gibberellinwhich in turn determines plant height. This particular enzyme namedas GA20 oxidase (GA20ox-2) catalyzes three key conversion stepsi.e. GA53 ïƒ GA44ïƒ GA19 ïƒ GA20 (Figure 3) during gibberellin biosynthesis process [3]. A removal of 280 bp within the coding regionof this GA20ox-2 gene [22] (results in mutation in the sd1 gene). Thismutation in turn leads to accumulation of GA53 in the plant there byhindering the conversion process. The mutant plant in experimentalconditions was found to be responsive to the externally supplied GA20suggesting that low level of gibberellin is the cause for this heightreduction. In rice, there is another GA oxidase gene coding for GA20ox-1 enzyme that occur in a different chromosome. Though GA20ox-1 and GA20 ox-2 are paralogous but their expression varies indifferent tissues. Therefore, although mutation in GA20 ox-2 affectsplant height but do not hinder grain yield in rice [3].
Figure 3: (One of the pathway showing Gibberellin biosynthesis in which GA 20-oxidase activity is necessary, redrawn after Qiao et al. [46]. Wild type sd1 gene in rice encodes for GA20 oxidase (GA20ox-2) enzyme that metabolizes bio-synthesis of the phyto-hormone gibberellin and this in turn determines plant height. This particular enzyme catalyzes three key conversion stepsi.e. GA53 ïƒ GA44ïƒ GA19 ïƒ GA20 during gibberellin bio-synthesis process.Mutation in sd1 gene leads to accumulation of GA53 in the plant there byhalting the conversion process and accounts for semi-dwarf feature [3].
Similar to rice, there is also a green revolution dwarf wheat variety.In case of green revolution wheat, dwarfing condition conferringsimilar benefit is due to a mutant allele at one of the Reduced height-1 loci [23]. Here, mutation leads to a gain-of-function transcriptionfactor that makes the plant unresponsive to Gibberellin hormoneand lead to reduced height [23]. The green revolution rice strikinglydiffer from that of the green revolution wheat both at genetic andbiochemical levels. Unlike green revolution rice, dwarfing in wheat is due to a semi-dominant gain-of-function mutation at one of thealleles encoding a DNA binding protein determining height of wheatplant.
Resistance to plant pathogen
Plant resistance to incoming pathogen community is mostlygoverned by gene for gene hypothesis which was proposed by Flor inearly 1940s [24]. According to the hypothesis, resistance in planta isgoverned by presence of R gene (for resistance) in the plant and an avrgene (for avirulence) in the pathogen. Absence of either of the genesor both the genes results in pathogenic condition. The hypothesis isnow a proven fact in many plant-pathogen interactions. The gene forgene hypothesis also predicts resistance as a dominant trait. However,there are evidences of plant-resistance being a recessive trait in several plant-pathogen interactions. The first elucidation of plant-resistance as a recessive trait was in barley which is conferred by an allele called mlo [25]. The molecular characterization of mlo allele suggested that wild type Mlo encodes a component that negatively regulates leaf cell death [26].
Interaction amongst rice and its bacterial pathogen Xanthomonasoryzae pv oryzae (Xoo) also follows the gene for gene hypothesis.Different R genes known in rice are Xa13, Xa17, Xa21, Xa26 etc.[27,28]. Out of these, resistance due to Xa13 and Xa17 are inheritedrecessively. Recent studies have unveiled role of wild type Xa13 intransport of sucrose from phloem parenchyma to apoplast region inrice leaves [29]. After gaining entry inside rice leaves, Xoo upregulates expression of these proteins by manipulating some of its elicitorssecreted via type III protein secretion system. An increased levelof these proteins ensures constant availability of sucrose inside thexylem and permits the pathogen to survive longer. Likewise, Xa17encoded protein also transports sucrose to phloem apoplast. In otherwords, these two genes function paralogously in rice and are namedas sweet proteins. Xoo cause disease in rice by deploying a kind ofeffectors called TALE (transcription activator like effectors) whichin turn upregulates Xa13 synthesis. Rice plant harboring mutatedXa13 will be unresponsive to Xoo TALE effectors and thus resistantto disease condition. Similarly, Xoo TALE effectors are also capable ofup regulating Xa17 synthesis leading to disease symptoms. Althoughit is known that Xoo resides in xylem and is able to deploy its type IIIeffectors for regulating gene expression in phloem parenchyma, themechanism by which the pathogen does it remain to be elucidated.Figure 4b depicts the phenomenon how pathogen entry andinduction leads to expression of sweet transporter proteins evenin mesophyll cells of leaf. Figure 4a shows the usual extracellularsucrose transport mechanism in the absence of pathogen [30].
Figure 4a: Schematic representation of the mechanism of sucrose transport from mesophyll cells to sieve element/companion cells in leaf, redrawn after Chen [30]. Sucrose produced photo-synthetically in the mesophyll cells is translocated within cells through the plasmodesmata and then to the phloem parenchyma cells. From phloem parenchyma cells sucrose is transported to extracellular milieu by SWEET sucrose effluxer, which are thought to bepresent in plasma membrane of the former. Following this, sucrose reachessieve element/companion cells via an ATPase driven H+-coupled sucrosesymporter SUT/SUC [30].
Figure 4b: Schematic representation of the mechanism by which pathogen gain access of sucrose synthesized in the mesophyll cells, redrawn after Chen [30]. Pathogen is able to induce expression of SWEET sucrose effluxers even in the mesophyll cells apart from phloem parenchymatous cells. In this way the pathogen receives nutrition inside the host [30].
In one report corresponding to Xa13, it has been said thatthe former also has a pivotal role in the pollen development indomesticated rice [31]. Mutations in the promoter region ofdominant Xa13 gene lead to down regulated expression that in turncause recessive state of the gene i.e. xa13. This condition leads to malesterility due to hindered pollen development and simultaneouslyconfers race specific resistance to bacterial blight pathogen [31].Presence of paralogs possibly compensate for the mutated dominantgenes.
Considering the important role of sweet transport proteins akinto Xa13 and other proteins in regulating pathogenesis mechanisms,research in this aspect carries lots of promises. Another xylem residentbacterial pathogen, Ralstonia solanacearum has also been shown toderive sucrose from the plant during infection process [32]. Whether recessive trait for sucrose transport function would have similarimpact on R. solanacearum pathogenesis too would be an intriguingquestion in plant-microbe interaction genetics. One stimulatingaspect is regarding the adaptability of both of these xylem pathogens.Xoo is a foliar pathogen, and R. solanacearum invades through rootfirst before spreading to entire plant system through xylem. How,both the pathogens are able to derive sucrose in this environmentmanipulating their type III protein secretion system elicitors will bean interesting comparative study between these two pathogens fortheir pathogenesis in near future.
Non seed-shattering trait in rice
Non-seed shattering trait of O. sativa is an important qualitythat has significantly influenced its domestication processes [33].Anthropogenic selection of the non-seed shattering rice variety overseed-shattering ones has gradually established the former. Geneticbasis of non-seed shattering trait has been connected with a numberof QTLs in rice genome such as sh3, sh4, sh6, sh8, qsh1, qsh2, qsh5,qsh11, qsh12, and sh-h [34-36]. More specifically a QTL sh4 encodesfor a transcription factor with varied functionalities [35b,37]. It hasbeen said that an alteration of a single nucleotide from G to T in thesh4 gene locus results in incomplete development and inadequateabscission zone function leading to non-shattering character of O.sativa [35b]. It is not known, if O. sativa possesses a paralog for thesame gene function in its genome. However, there is no effect on theproductivity of the rice cultivar with this sh4 mutation, similar toother features discussed above. Although, there are different viewswith respect to the significance of sh4 for the non-shattering featurein rice [38], but, recessive state of sh4 carrying this alteration hascertainly been shown to have prominent effect on the non-shatteringtrait of domesticated rice [35b]. Perhaps, with time, more number ofQTLs may be discovered, and it is possible that a cumulative effectof all these QTLs might be necessary for the feature discussed above.
Discussion
Recessive traits discussed here are due to mutations in genessuch as BADH2, sd1, Xa13 and seed-shattering loci that encodesessential functional ingredients in plant systems. For instance, functional gene products such as betaine aldehyde dehydrogenasefacilitate plant’s tolerance to stress; GA20ox-2 catabolizes gibberellinsynthesis, and sweet protein Xa13 is involved in transporting sucrosefrom photosynthetic to non-photosynthetic tissues via phloem.Seed-shattering locus such as sh4 accounts for proper developmentof abscission zone between grain and pedicel that renders efficientseed dispersal in wild rice. Nevertheless, non-functional products ofmutated states of these genes also exist. Question would arise, thenby what means plants counteract mutations in these essential genes?In actuality, plants harbor additional members of these gene familiescompensating for mutated copies, as is seen in case of rice. In rice,Xa17 serve as an alternative sweet protein like that of Xa13. Sameway, GA20oxidase-1 functions alike GA20oxidase-2, although expressionsare tissue-specific. Likewise, BADH1 can compensate for BADH2functions. The additional copies as mentioned above help the plant tosurvive and grow normally like their wild type counter parts. Unlikein all other three genes discussed here, paralogs for seed-shatteringfeature is yet unknown in rice. How the mutations in seed-shaterringloci are counterbalanced in the domesticated rice is an intriguingtheme to delve into.
Generally, persistence of recessive traits in domesticated ricevarieties retaining high yielding attribute is an un-anticipatedoccurrence. In case of a rice plant which harbor mutant gene forGA20 oxidase-1 may also have short-stature due to intact GA20 oxidase-2activity but its productivity may not resemble with that of GreenRevolution rice. These observations may compel us to assume thatgenes whose paralogs are present in a genome may attribute torecessive traits in the organism through their differential expressionpatterns. It is axiomatic that due to lethality, recessive traits are noteasy to be identified in plants. In this context generation of haploidplants of rice through tissue culture technique is going to be useful inidentification of beneficial recessive traits in rice. Ploidy in crop plantsis going to be important for recessive traits. For instance, diploidynature of crops like rice, maize and barley has been shown to behandy in identifying nature of quantitative traits [39-41]. However,genetic analysis in wheat is tricky, due to its hexaploid nature and alsocomparatively larger genome. This has effected several limitations instudying QTLs in wheat [42,43]. Unlike rice, it is least expected thatwheat would have several recessive genes bestowing advantages. But,interestingly, recessive state of gene conferring pathogen resistancein wheat has also been known [44,45]. Pathogen intervened stressmay have contributed greatly toward selection and perpetuation ofrecessive resistance genes in wheat and concurrently anthropogenicselection must have played vital role too. There is another rivetingfact about recessive trait. Genes whose recessive state confersdistinctive advantages to the host were actually not evolved for suchfunctions. Rather, features due to recessive state of genes could beemployed for benefit by the host. For instance evolution of BADH2was not actually for fragrance neither was Xa13 evolved for protectiveresponses [46,47].
Conclusion
Interestingly, if an organism such as plant can survive, grow andsustain without function of a gene i.e. in absence of a functional gene,then, evolution of that particular gene function or the trait appears to be paradoxical! We assume that the functional alleles of these traitswere selected in rice during the growth under natural conditionsbut now due to anthropogenic selection, which is basically a nonnaturalmode of selection, rice plant species with traits due to nonfunctionalgene(s) have been established. For instance, fragrance inrice might have been deleterious for the plant to survive well as itcould invite potential predators of grain and the plant such as insectsunder natural conditions. Similarly tall rice plants have advantageto grow in low land areas. In the same way, sucrose transporterproteins like Xa13, Xa17 have important role in the rice plant duringnutrient-deficient conditions, as these provide sucrose to importantand metabolically active regions in the plant! Incidentally, non-seedshattering feature in the domesticated rice gives advantage overshattering ones by allowing for efficient harvesting as well as storage.Unconsciously, mutations in the shattering loci of wild rice varietieshelped anthropogenic selection and non-seed shattering feature gotestablished. Furthermore, in due course of evolution, worth of theserecessive traits might have been in devising a selection mechanismfor giving rise to polymorphic alleles. The selective advantages ofthese traits imply that the polymorphisms evolved in these genes arein compliance with the selection theory of evolution. In the comingdays, we anticipate sufficient evidence for drawing a connectionbetween recessive trait and polymorphism in actuality.
Acknowledgement
SKR is thankful to his students with whom he discussesevolutionary genetics. AB is thankful to UGC, Govt. of India forJunior Research fellowship grant. We thank Mr. Manas Jyoti Kalitafor helping us with the sketch work.
References
- Frary A, Nesbitt TC, Grandillo S, et al. (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289: 85-88.
- Bhattacharjee P, Singhal RS, Kulkarni PR (2002) Basmati rice: a review. Int J Food Sci Tech 37: 1-12.
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, et al. (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416: 701-702.
- Fitzgerald MA, Mccouch SR, Hall RD (2009) Not just a grain of rice: the quest for quality. Trends Plant Sci 14: 133-139.
- Kovach MJ, Calingacion MN, Fitzgerald MA, Mccoucha SR (2009) The origin and evolution of fragrance in rice (Oryza sativa L.). Proc Natl Acad Sci U S A. 106: 14444-14449.
- Pramnoi P, Somta P, Chankaew S, Juwattanasomran R, Srinives P, et al. (2013) A single recessive gene controls fragrance in cucumber (Cucumis sativus L.). J Genet 92: 147-149.
- Purugganan MD, Fuller DQ (2009) The nature of selection during plant domestication. Nature 457: 843-848.
- Kadam BS, Patankar VK (1938) Inheritance of aroma in rice. Chronica Botanica 4: 32..
- Jodon NE (1944) The inheritance of flower fragrance and other characters in rice. J Am Soc Agron 36: 844-848.
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, et al. (2005) Cytokinin oxidase regulates rice grain production. Science 309: 741-745.
- Fan C, Xing Y, Mao H, Lu T, Han B, et al. (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112: 1164-1171.
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX, et al. (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39: 623-630.
- Bai X, Wu B, Xing Y (2012) Yield-related QTLs and their applications in rice genetic improvement. J Integr Plant Biol 54: 300-311.
- Uga Y, Yamamoto E, Kanno N, Kawai S, Mizubayashi T, et al. (2013) A major QTL controlling deep rooting on rice chromosome 4. Sci Rep 3: 1-6.
- Bradbury L, Gillies S, Brushett D, Waters DL, Henry RJ, et al. (2008) Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol Biol 68: 439-449.
- Chen S, Yang Y, Shi W, Ji Q, He F, et al. (2008) Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 20:1850-1861.
- Buttery RG, Ling LC, Juliano BO (1982) 2-Acetyl-1-pyrroline: an important aroma component of cooked rice. Chem Ind (London): 958-959.
- Paule CM, Powers JJ (1989) Sensory and chemical examination of aromatic and non-aromatic rices. J Food Sci Technol 54: 343-346.
- Jiamsomboona K, Treesuwanc W, Boonyalai N (2012) Dissecting substrate specificity of two rice BADH isoforms: Enzyme kinetics, docking and molecular dynamics simulation studies. Biochimie 94: 1773-1783.
- Jennings PR (1964) Plant type as a rice breeding objective. Crop Sci 4: 13-15.
- Walcott JJ, Laing DR (1976) Some physiological aspects of growth and yield in wheat crops: a comparison of a semi-dwarf and a standard height cultivar. Austral J Exp Agric Anim Husb 16: 578-587.
- Spielmeyer W, Ellis MH, Chandler PM (2002) Semidwarf (sd-1), ‘‘green revolution’’ rice, contains a defective gibberellin 20-oxidase gene. PNAS 99: 9043-9048.
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, et al. (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400: 256-261.
- Flor HH (1955) Host-parasite interaction in flax rust- its genetics and other implications. Phytopathology 45: 680.
- Jorgensen JH, Wolfe M (1994) Genetics of powdery mildew resistance in barley. Crit Rev Plant Sci 13: 97-119.
- Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, et al. (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 88: 695-705.
- Cao Y, Ding X, Cai M, Zhao J, Lin Y, et al. (2007) The expression pattern of a rice disease resistance gene Xa3/Xa26 is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics 177: 523-533.
- Antony G, Zhou J, Huang S, Li T, Liu B, et al. (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 22: 3864-3876.
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, et al. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527-534.
- Chen LQ (2014) SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol 201: 1150-1155.
- Chu Z, Yuan M, Yao J, Ge X, Yuan B, et al. (2006) Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev 20: 1250-1255.
- Jacobs JM, Babujee L, Meng F, Milling A, Allen C, et al. (2012) The in planta transcriptome of Ralstonia solanacearum: conserved physiological and virulence strategies during bacterial wilt of tomato. mBio 3:e00114-12.
- Subudhi PK, Singh PK, DeLeon T, Parco A, Karan R, et al. (2014) Mapping of seed shattering loci provides insights into origin of weedy rice and rice domestication. ‎J Hered 105: 276-287.
- Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, et al. (2006) An SNP caused loss of seed shattering during rice domestication. Science 312: 1392-1396.
- Li C, Zhou A and Sang T (2006) Genetic analysis of rice domestication syndrome with the wild annual species, Oryza nivara. New Phytol 170: 185-194.
- Li C, Zhou A, Sang T (2006) Rice domestication by reducing shattering. Science 311: 1936-1939.
- Ji H, Kim SR, Kim YH, Kim H, Eun MY, et al. (2010) Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J 61: 96-106.
- Yan H, Ma L, Wang Z, Lin Z, Su J, et al. (2015) Multiple tissue-specific expression of rice seed-shattering gene SH4 regulated by its promoter pSH4. Rice (N Y) 8: 12. Doi: 10.1186/s12284-015-0047-4.
- Zhu Y, Ellstrand NC, Lu B (2012) Sequence polymorphisms in wild, weedy, and cultivated rice suggest seed-shattering locus sh4 played a minor role in Asian rice domestication. Ecol Evol 2: 2106-2113.
- Abler BSB, Edwards MD, Stuber CW (1991) Isoenzymatic identification of quantitative trait loci in crosses of elite maize. Crop Sci 31: 267-274.
- Xiao JH, Li L, Yuan LP, Tanksley SD (1996) Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from a subspecific cross. Theor Appl Genet 92: 230-244.
- Tinker NA, Mather DE, Rossnagel BG, Kasha KJ, Kleinhofs A, et al. (1996) Regions of the genome that affect agronomic performance in two-row barley. Crop Sci 36: 1053-1062.
- Hyne G, Snape JW (1991) Mapping quantitative trait loci for yield in wheat. In: Pesek J, Herman M, Hartmann J (ed.): Biometrics in plant breeding, Proceedings of the 8th meeting Eucarpia section biometrics on plant breeding, Brno, pp: 47-56.
- Berke TG, Baenziger PS, Morris R (1992) Chromosomal location of wheat quantitative trait loci affecting agronomic performance of seven traits, using reciprocal chromosome substitutions. Crop Sci 32: 621-627.
- Biffen RH (1905) Mandel’s laws of inheritance and wheat breeding. J Agr Sci 1: 4-48.
- Gupta AK, Saini RG, Gupta S, Malhotra S (1984) Genetic analysis of two wheat cultivars, 'Sonalika' and 'WL 711' for reaction to leaf rust (Puccinia recondita). Theor Appl Genet 67: 215-217.
- Buttery RG, Ling LC, Juliano BO, Turnbaugh JG (1983) Cooked rice aroma and 2- acetyl- 1-pyrroline. J Agric Food Chem 31: 823-826.
- Quiao F, Yang Q, Wang C, Fan YL, Wu XF, et al. (2007) Modiï¬cation of plant height via RNAi suppression of OsGA20ox2 gene in rice. Euphytica 158: 35-45.