Rapid and Highly Competent Shoot Regeneration of Pigeon Pea (Cajanus Cajan) using Variable Explants Via In Vitro Culture System
Ramdas V Raut1, Gaurav A Dhande2*, Jaysing C Rajput3 and Arun G Ingale4
1,2,3Nirmal Seeds Pvt. Ltd, Bhadgaon Road, Pachora, Dist. Jalgaon 424201 (M.S.) India
4Department of Biotechnology, School of Life Science, North Maharashtra University, Jalgaon-425001 (M.S.) India
Corresponding author: Gaurav A. Dhande, Nirmal Seeds Pvt. Ltd, Bhadgaon Road, Pachora, Dist. Jalgaon 424201 (M.S.)India, Tel: +91-9422212452; E-mail: gadhande@nirmalseedsindia.com
Citation: Raut RV, Dhande GA, Rajput JC, Ingale AG. Rapid and Highly Competent Shoot Regeneration of Pigeon Pea (Cajanus Cajan) using Variable Explants Via In vitro Culture System . J Plant Sci Res. 2015;2(2): 132.
Copyright © 2015 Raut RV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 2, Issue: 2
Submission: 24/08/2015; Accepted: 30/09/2015; Published: 05/10/2015
Abstract
Pigeon pea is legume crop play a crucial role as source of dietary protein in diet, growing extensively in the rainfed and dryland spots of India andworldwide. Plant tissue regenerate through in-vitro system attempting organogenesis as well as embryogenesis pathway, which are in support of unfamiliargenes assimilation targeted for development of transgenic plants. Present study was undertaken to investigate the most appropriate explant type in Pigeonpea regeneration by virtue of in vitro culture system. Genotype Durga (NTL-30) was breed and used as principal material for regeneration studies. Explantsisolation from in vitro elevated germinating 6-8 days old seedlings were used for embryonic axis and cotyledonary node, whereas isolation of Scutellum (IZE)explants from overnight imbibed seed. Isolated explants were cultured on Murashige and Skoog medium supplemented with zeatine (0.572, 1.35, 1.47, 2.32μM) and kinetin (0.46, 0.93, 1.39 & 1.86 μM) as plant growth regulator for regeneration. Additionally medium incorporated with 2.94 μM of silver nitrate toaccelerate growth. Successive to regeneration, shoots bud originated from embryonic axis, cotyledonary node and Scutellum were rapidly elongated on MSmedium included 0.50 μM gibberlic acid and further rooted on M.S. medium cantoning idol- acetic acid 0.57 μM which notice 95% efficient root induction.The methodology reported here is very simple, skilled and reproducible, can be employing diagonally to corresponding genotypes of pigeon pea, thus canbe practically useful for the establishment of transgenic plants.
Keywords: Cajanus cajan; Shoot regeneration; In vitro culture system; (IZE)
Abbreviations: IZE: Immature zygotic embyo; M.S.: Murashige and Skoog; AgNo3: Silver Nitrate; BAP: 6-Benzylaminopurine; GA3; Gibberlicacid; NTL: Nirmal Tur line; IAA: Indol acetic acid
Introduction
Pigeon pea widely well-known as red gram and locally “tura” inIndia, it is most significant food legume having crucial major grainlegumes of the semi-arid tropics. It is grown commercially throughoutthe globe and cultivated about fifty countries of Asia, Africa, andAmerica for food, fodder, fuel, soil conservation, and green manure [1]. The productivity of pigeon pea are introverted by numerousdiseases, including sterility mosaic, fusarium wilt, Phytophthorablight, Alternaria blight and major insect pest Helicoverpa armigera[2]. Biotechnological application such as genetic transformationheaded for superior pest resistance put forward opportunities forrapid improvement of pigeon pea. However the accessibility ofin vitro regeneration method is a prerequisite for effective plantregeneration. Regeneration through in vitro culture for exploitationof plant cell totipotency confirm organogenesis and regeneration fromvarious explants type of pigeon pea has been reported earlier [3,4].Regeneration of pigeon pea via organogenesis has been reported with pre-existing apical meristems [6], cotyledonary node [5] embryonicaxis [6].
The above published literatures suggest that addition ofcytokinine in regeneration medium could be the underpinning forpigeon pea shoot bud discrimination [7]. Silver nitrate as an additivein medium promotes plant growth regulation and morphogenesisin recalcitrant crop such as pigeon pea. Participating silver ionsin the form of nitrates as primary role and influencing somaticembryogenesis. Efficient shoot and root development which is theprimary prerequisites for successful genetic transformation, reportedearlier [8]. Hence forth the aim of the present research to fill up theseprospective of shoots regeneration, proliferation and in vitro plantletsdevelopment. Pigeon pea regeneration through in vitro by addition ofhormonal combination reported is rapid, effortless and efficient forregeneration of Cotyledonary node, embryonic axis and Scutellum(IZE) explants.
Materials and Methods
Plant material
Healthy seeds of Pigeon pea Durga (NTL-30) were obtainedfrom Nirmal Seeds Pvt. Ltd., Pachora, Jalgaon, (M.S.) India. Matureuniform seeds were surface sterilized by rinsing 2-3 times withdistilled water, followed by treatment with solution of concentratedH2SO4 (v/v) for two minute in favour of slacken the seed coat, seedswere then rinsed thoroughly with sterile distilled water. The seed wereconsequently transferred in 2% bavistine for 60 minute incubationperiod; the seeds were thereafter rinsed with sterile distilled water.Subsequently seeds were treated with 0.01% HgCl2 for 30 minute andwash severely again with distilled water. Seeds were air dried andgerminated on MS half strength basal medium supplemented withBAP 8.90 μM. The pH of the medium was adjusted 5.8 and sterilizedat 1210C for 15 minute. Seeds were incubated at room temperature(27 ± 1 °C) for 16 hours photo-period.
Explant preparation
Explants were prepared from six to eight days old in-vitrogrown germinating seedlings. Embryonal axis isolation from basalswollen region of the excised seedling by using germinating seedling.Cotyledonary node isolated from apical region of seedling by incisingboth cotyledonary leaf from base and apical primary leaf (Figure 1).
Figure 1: Cotyledonary node exlants (a), embryonic axis explants (b) fromgerminating seedlings (GS).
Scutellum/immature zygotic embryo (IZE) explant were isolatedfrom overnight imbibed surface sterilized healthy seeds dried usingsterile filter paper, seed test were removed and exposing the zygoticimmature embryo and cotyledons. The cotyledons were separatedby slight incision from central axis developing immature zygoticembryo. Nourishing tissue with zygotic embryo was used as thestarting explants material for regeneration. Isolated explants weretransferred in MS liquid medium without drying (Figure 2).
Figure 2: Scutellum explants preparation stages A) whole seed B) Cotyledon excise from axis, C&D) Scutellum with attached cotyledon E) Scutellum.
Shoot bud Regeneration
In order to regenerate shoots of Pigeon pea cultivars Durga (NTL-30) through organogenesis using embryonic axis, cotyledonary nodeand Scutellum , explants were cultured on shoot induction MS medium supplemented with 58.8 μM AgNO3 [9]. The various concentration ofplant growth regulator zeatine 0.57, 1.35, 1.47, 2.32 μM and kinetin0.46, 0.93, 1.39, 1.86 μM in combination as well as solitary were tried.The optimal concentration for shoot regeneration integrates withzeatine 1.35 μM and kinetin 0.93 μM at highest efficiency. The pH ofthe medium was adjusted to 5.8 before autoclaving and medium wassolidified by 0.8% Agar-agar. Explants were incubated at 25 ± 2 °Cunder l6 hour’s photo-period for two week. Shoot bud were elongatedon elongation medium containing GA3 0.50 μM. After incubation,the responses of shoots elongation were recorded and the elongatedshoots were transfer on root induction medium supplemented withIAA-0.57 μM. The abundant grown rooted plants were ultimatelyplanted in coco peat filled in small pots supplemented with enoughwater and 65 % humidity in growth room conditions for 15 -20 days,thereafter acclimatize in glass house for further adaptation.
Results and Discussion
Seed germination
The seed germination on half strength MS medium having 8.90μM BAP was exposed enhancing rate of germination elevated up to95 percent. Benzylaminopurine prominently facilitate swollen basalsegment of seedling and leads to developed embryonic axis whileabsence of BAP deformation of embryonic axis explants. Utilizationof concentrated sulphuric acid during germination slackens theseed coat and condenses the elevation of infectivity parallel workspreviously have been reported [10].
Regeneration
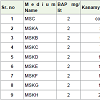
In the present study zeatin and kinetin combination was foundto be appropriate for shoot induction and proliferation. Frequencyof shoot regeneration was found to be highest in embryonic axis,followed by cotyledonary node and Scutellum explants (Table 1).Accumulation of AgNO3 2.35 μM, 2.94 μM, 4.70 μM, 5.88 μM wereattempt to examine the effect on shoot regeneration response (Table 2) and it was found that optimum 2.94 μM support the maximummultiple shoot development (Figure 3).
Table 2: Effect of PGR concentration zeatin and kinetin on shoot bud induction and contributory role of AgNO3 from embryonal axis, Cotyledonary node and Scutellumexplants of pigeon pea.
Figure 3a,3b and 3c: 3a: Embryonal axis explants (i) shoot development (ii) 3b: Cotyledonary node explants (i) shoot development (ii) 3c: Imature zygotic embryo (Scutellum) explants (i) shoot develpment(ii).
Embryonic axis, cotyledonary node and Scutellum explants wascultured on MS medium (MSZKb) supplemented with zeatin 1.35μM and kinetin 0.93 μM alongside 2.94 μM AgNO3 shown 96% shootregeneration with shoot length of 2.0-4.8 cm recorded (Table 2). Alonezeatine as well as kinetin unable to influence efficient shoot formation,however in combination it facilitate highest efficiency headed forshoots proliferation passing through silver nitrate. The intermissionof shoot elongation was overcome by shifting to a shoot elongationmedium contain GA3 0.50 μM shows distinctly lengthen shoots (Table 3). Elongation medium proficient for plantlets enlargement with 89percent and shoot length of 4.10-4.8 cm was reported (Figure 4). Themaximum frequency of shoot formation was 74 to 96 percent onregeneration medium (Table 1). The lowest regeneration frequencyhas shown 48 to 60 percent, the regeneration frequency superior inthe concentration of lesser altitude of cytokinine is optimal for shootbud formation. Higher altitude concentration of cytokinine above 3μM shows shoot frequency decline drastically.
Root development and hardening
Elongated Shoots 3.5 to 4.0 cm extended was relocating to rootinduction medium supplemented with indol-3 acetic acid 0.57 μM(Table 3). Regenerated plantlets with sprouted roots (Figure 5) weretransfer to pot filled with coco peat in growth room conditions at65% humidity for three week subsequently acclimatisation in glasshouse for further adaptation.
Embryonic axis, cotyledonary node and Scutellum explantsregeneration was focussed, extremely simple and less time consumingdevelopment towards huge number of plantlets. Regenerated Plantsproduced was true to phenotype with insignificant rate of somaclonalvariation and chromosomal abnormalities. Embryonic axis explantsreveal that profuse shooting at elevated rate and more appropriatesince meristematic cells are premeditated have report in Vigna mungo[9], hence employed to accomplish higher rate regeneration and sameresults were obtained. Expereiment results conclude embryonic axisproduced a bunch of shoot bud hence it is the most amiable explantsfor regeneration (96%). Cotyledonary node is the second prior explant for producing mass of multiple shoot at the auxiliary budregion therefore considered as more appropriate for regeneration.Considering pigeon pea is recalcitrant nature both Cotyledonarynode and embryonic axis achieved contemporary 80% regenerationresponse.
Immature zygotic embryo (IZE) tenure as Scutellum was thethird preferably explants for generating high frequency for shootregeneration induced by exogenous auxin to proliferate by meanof somatic embryogenesis is report in maize [11]. On the contraryallowing for fruitfully producing maximum plantlets and observed74% regeneration during contemporary observation.
Plants behavioural study for regulating morphogenesis usuallyby hormonal balance like a key factor in vitro culture system .Theoptimum hormones combination ratio facilitate to influence plantenzymatic reactions resulting of plant growth and important tothe particular effect on growth and morphological amendmentproven [12]. Existing result also indicated that the regeneration andelongation of shoot was promoted by equilibrium of cytokinine.In the existing study hormone combination of zeatin 1.35 μM andkinetin 0.93 μM in MS medium generate better for shoot regeneration (96%). The best possible level of hormonal combination zeatin andkinetin support the highest number of shoot bud proliferation, noreport has been published yet with these combinations. Elongationof regenerated shoot found 4.80 cm in MS medium containing 0.50μM GA3 and revealed 85 percent enlargement. Establishment ofroots towards elongated shoots is essential for successful plantlets inthe soil. As per the requirement of plantlets adaptation in soil withprofuse rooting cotyledonary node and embryonic axis show highestrooting frequency (95.0%) in presence of 0.50 μM of IAA.
Additionally to the medium silver nitrate incorporate as wellconsidering adverse effect on shoot induction in vitro by recognizedto promote multiple shoot formation and adsorbing phenoliccompound in the medium to inhibit the division of cell in differentplants. In vitro shoot formation was improved by incorporating silvernitrate in the culture medium reported [13]. Low concentrationAgNO3 was found to cause delayed senescence resulting in improvedgrowth of the proliferated shoots. Silver nitrate was found to bebeneficial in the regeneration peanut [14], cow pea [15]. Ongoingresults show incorporation of 2.94 μM AgNO3 significantly enhancesshoot proliferation.
Conclusion
The novelty of the cited research shows that combination ofcytokinine i.e. zeatin and kinetin responded vigorously to shootinduction for cotyledornary node, embryonic axis and Scutellumexplants, which was earlier reported incredibly time consumingthrough a variety of auxin and cytokinine combination. The shoot proliferation with silver nitrate as additives expose at superiorvelocity. The above three descibed explants work out on singlehormonal combination for regeneration and no reports werefound with such single-handedly combination. Root inductionwas produced within little time period of which is prerequisite forhardening. This was the first report to employ silver nitrate alongwith cytokinine combination which facilitates regeneration withelevated velocity within stipulated time. Current protocol is based onregeneration of shoot from the embryonic axis, cotyledornary nodeand Scutellum with high competency and is highly appropriate forgenetic transformation.
Acknowledgment
The authors would like to thank to the Managing Director,Shri. R.O. Patil of Nirmal Seeds Pvt. Ltd. for providing facilities andassistance.
References
- Krishna G, Reddy PS, Ramteke PW, Pogiri R, Sayed SS (2011) In vitro regeneration through organogenesis and somatic embryogenesis in pigeon pea ( Cajanus Cajan(L.). Physiol Mol Biol Plants 17: 375-385.
- Reddy MV, Sharma SB, Nene YL (1990) Pigeon pea: disease management. In: Nene YL, Hall SD, Sheila VK (Eds) the pigeonpea. CAB, Wallingford, UK. 303-347.
- Shrinivasan T, Verma V, and Kirti P (2004) Efficient shoot regeneration in pigeon pea, Cajanus cajan (L) Millisp. using seedling petioles. Current science 86: 1.
- Shiva Prakash N, Pental D, Bhalla SN (1994) Regeneration of pigeon pea (Cajanus cajan) from cotyledonary node via multiple shoot formation. Plant Cell Rep 13: 623-627.
- Geetha N, Venkatachalam P, Prakash V, Lakshmi Sita G (1998) High frequency induction of multiple shoots and plant regeneration from seedling explants of pigeon pea (Cajanus cajan L.). Curr Sci 75: 1036-1041.
- Krishna G, Reddy PS, Ramteke PW, Bhattacharya PS (2010) Progress of tissue culture and genetic transformation research in pigeon pea [Cajanus cajan (L.) Millsp.]. Plant Cell Rep 29: 1079-1095.
- Dayal S, Layanya M, Devi P, Sharma KK (2003) An efficient protocol for shoot regeneration and genetic transformation pigeonpea [Cajanus cajan (L) Millsp] using leaf explants. Plant Cell Rep 21: 1072-1079.
- Bais HP, Sudha GS, Ravishankar GA (2000) Putrescine and AgNO3 influences shoot multiplication, In vitro flowering and endogenous titres of polyamines in Cichorium intybus L cv Lucknow Local. J Plant Growth Regul 19: 238-248.
- Ignacimuthu S, Franklin G (1999) Regeneration of plantlets from cotyledon and embryonal axis explants of Vigna mungo L. Hepper. Plant Cell, Tissue and Organ Culture 55: 75-78.
- Lawrence PK, Koundal KR (2001) Agrobacterium tumefaciens-mediated transformation of pigeon pea (Cajanus cajan L. Millsp.) and molecular analysis of regenerated plants. Current science 80: 1428-1432.
- Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, et al. (2002) Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 129: 13-22.
- Satyavathi VV, Prasad V, khandelwal A, Shaila MS, Sita GL (2002) Expression of hemagglutinin protein of Rinderpest virus in transgenic pigeon pea [Cajanus cajan (L.) Millsp.] Plants. Plant Cell Rep 21: 651-658.
- Ganesh SD, Sreenath HL (1996) Silver nitrate enhanced shoot development in cultured apical shoot buds of Coffea arabica Cv Cauvery (S4347). Journal of Plantation Crops 24: 577-580.
- Pestana MC, Lacorte C, de Freitas VG, de Oliveira DE, Mansur E (1999) In Vitro Regeneration of Peanut (Arachis Hypogaea L.) through Organogenesis, Effect of Culture Temperature and Silver Nitrate. In Vitro Cellular & Developmental Biology-plant 35: 214-216.
- 15.Brar MS, Moore MJ, Al-Khayri JM, Morelock TE, Anderson EJ. (1999) Ethylene inhibitors promote in vitro regeneration of cowpea (Vigna unguiculata L.). In Vitro Cell Dev Biol Plant 35: 222-225.