Protoplast Isolation and Callus Formation in Oroxylum Indicum (L.) Vent
Mamta Gokhale*
Corresponding author: Dr. Mamta Gokhale, Department of Biotechnology, St. Aloysius College (autonomous), Jabalpur- M.P, India,; E-mail: mamtashrirang@gmail.com
Department of Biotechnology, St. Aloysius College (autonomous), Jabalpur-M.P, India
Citation: Gokhale M. Protoplast Isolation and Callus Formation in Oroxylum Indicum (L.) Vent. J Plant Sci Res. 2015;2(1): 119.
Copyright © 2015 Mamta Gokhale. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Plant Science & Research | ISSN: 2349-2805 | Volume: 2, Issue: 1
Submission: 19/02/2015; Accepted: 23/03/2015; Published: 30/03/2015
Abstract
A simple protoplast isolation protocol from leaf of a medicinally important endangered tree Oroxylum indicum (L.) Vent. was designed to recover totipotent plant protoplasts. The key elements of the protocol are, use of protoplast releasing enzymes (cellulase, pectinase and hemicellulose) alone and in combination in different concentrations for different incubation period of time. Study revealed thatprotoplast yield was obtained maximum (6x105) in the 13S CPW provided with two combinations of enzymes (hem1+ cel 0.1+pec 0.1 and hem1+ cel 0.5 + pec 0.5).Whereas maximum protoplast viability (70.51%) obtained on hem1+ cel 0.1+pec 0.1 (13S CPW). The optimum time of incubation was observed 4 hrs (25 ± 1°C temp). The isolated protoplast grown gradually when the suspension of protoplast was inoculated on MS media containing (BAP 1 mgL-1and AgNO3 2 mgL-1). The protocol has ensured reproducibility, higher yields and is gentle, as the protoplasts obtained were amenable to cell wall regeneration and cell division. Plantlets were regenerated form the callus developed from isolated protoplast.
Keywords: Protoplasts; Oroxylum indicum (L.) Vent.; Enzymes, Totipotency, Callus, Plantlets
Introduction
The development of protoplast systems has increased theversatility of plans for use in both biochemical and genetic research.They have become indispensable tools in genetic engineering andcrop breeding of all the possible starting points for plant geneticmanipulation, only protoplasts offer the opportunity to takeadvantage of all the technologies now available. Studies on protoplastisolation conditions have identified various parameters, includingenzyme combination, the choice of the explant and its physiologicalstate within a range of plant species [1].
Oroxylum indicum (family- Bignoniaceae) also known as ‘Sonapatha’ is an important herb in Ayurvedic medicine and indigenous medical system for over thousands of years [2]. It is considered as endangered due to over exploitation. Oroxylum indicum has been used as a single drug or as a component of certain polyherbal drug preparations in Indian system of medicine i.e. Ayurveda. It is an active ingredient of well-known Ayurvedic formulations like Chyavanprash, Dashmularistha [3]. Protoplast isolation and regeneration from Oroxylum may open new vistas in the area of biotechnological experimentation. Methods of protoplast isolation with too many steps involved, often result in the introduction of cell contamination at some stage or the other. Here, we present a simple method in which different incubation period of time and different combinations of enzymes (cellulase, pectinase and hemicellulose) that have functioned synergistically to release protoplasts with relative ease in Oroxylum indicum (L.) Vent. The isolated protoplast was further subjected to callus formation and plant regeneration.
Material and Methods
Source tissue
Tissue for protoplast isolation consisted of fully expanded leaves excised from greenhouse-nature grown plant leaves of Oroxylum indicum (L.) Vent.
Explant preparation prior to enzyme exposure
Leaves of field-grown plants were thoroughly washed in running water for about 10 min, surface sterilized with 0.1 % HgCl2. Surface decontaminated leaves of were chopped into 2-3 mm strips. All tissues were weighed in sterilized petriplates for fresh weight determination before they were subjected to enzyme digestion.
Protoplast isolation, purification and culture
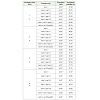
The protoplast isolation solution consisted of Cell Protoplast Washing (CPW) media (Table 1) was prepared with 13M (Mannitol) and 13S (Sucrose). The quantity and the ratios of enzymes required for protoplast release were determined. Different concentrations (0.1%, 0.5% and 1%) of digesting enzymes cellulase, pectinase and hemicellulase (1%) were prepared with single enzyme or in combination in CPW medium consisted with 13 M (Mannitol) and filter sterilized. Conditions of temperature and incubation time were optimized with these enzymes. To identify factors that might influence the efficiency in terms of protoplast release, rapidity and viability, incubation of leaf tissue was earned out at different temperatures.
Procedure for protoplast release
Surface sterilized leaves of O. indicum. were peeled and soaked in 13M CPW for 1 hr in dark at 25 ± 1°C. Leaf tissues transferred to 10 ml of filter sterilized enzyme solutions (in 13S CPW) and incubated in dark for different time intervals. To determine the time required for complete release of protoplasts at incubation temperatures 25 ± 1°C, aliquots were microscopically examined at different time intervals i.e.2 h, 4 h, 6 h, 8 h. Enzyme solution replaced with 13S CPW solutions. Digested leaf pieces were squeezed in 13S CPW solutions without enzymes. The density of protoplast isolated from different enzymatic treatment and incubation time was counted with haemocytometer. Yield of the protoplast was calculated by
Y = n x 103 x X
Where
n= number of protoplasts counted in 5 triple ruled squares.
X = volume of protoplast suspension.
After knowing the yield/fixed volume, the desired densityof protoplast in suspension culture was adjusted i.e., 1 x 10 5 byeither dilution (adding more medium) or concentration of existingsuspension (by centrifugation and removal of supernatant).
Protoplast culture: Aliquots of purified protoplasts were plated in 10 ml of solid MS (Murashuge & Skoog 1962) medium supplemented with growth regulators (BAP 1 mgL-1and AgNO3 2 mgL-1) into 60 x 15 mm glass petriplates. As this media combination has been proved appropriate for regeneration of plantlets from both direct and indirect method [4,5]. The plating density was adjusted to obtain 2 x 104 protoplasts per ml of medium. The cultures were initially incubated in dark at 25 ± 2°C temperature for two weeks and then shifted to alternating light (of intensity 200 μEm-2 s-1) and dark regimes of 14 and 10 h respectively. Samples were tested periodically for viability and wall regeneration.
Results
The results obtained from the various protoplast isolation experiments performed are summarized in Table 2. With the method described now, tissues of leaves of O. indicum explanted were most readily acted upon by the enzymes. A microscopic examination carried out following digestion showed that the protoplast release was complete. All experiments designed and investigated yielded protoplasts. Simultaneous release of protoplast is shown in Figure 1. Green, round protoplast bodies of O. indicum were isolated from mesophyll cells of leaf (Figure 2A-2B).
Table 2: Effect of digesting enzymes & incubation time on yield and viability of protoplast in Oroxulum indicum (L.) Vent.
It is clear from the Table 2 that a combination of enzymes has a visible effect on the release of protoplast from leaf tissue. A combination of enzymes hemicellulose 1%, cellulose 0.1% and pectinase 0.1% (in 13S CPW), was capable to yield maximum protoplast yield (6x105) with maximum protoplast viability (70.51%) for 4 hrs of incubation period. The isolated protoplast grown gradually, when the suspension of protoplast was inoculated on solid MS media containing (BAP 1 mgL-1and AgNO3 2 mgL-1) in petriplates (Figure 3A). Growth of protoplast is visible in the form of green patches, which was increased gradually after different time intervals (4days, 8 days, 12 days) (Figure 3B). Gradually the isolated protoplast clusters have been developed into soft, live callus, when transferred on same media composition in test tubes. After 45 days fully grown callus was obtained (Figure 4).
Figure 3: Growing cluster of protoplasts on MS media (BAP 1 mgL-1andAgNO3 2 mgL-1 after 12 & 24 days of inoculation.
Discussion
Digestion of explants for protoplast release, carried out by another method [6], required a longer time (8-12 h) on elevated digestion temperature for protoplast release. The yield of protoplasts was also comparatively low. The present protocol provides a simple and an easy-to-handle procedure that ensured satisfactory yields and quick recovery of viable protoplasts from the leaf of 10 years old tree of O. indicum. The protocol is simple in that the various pretreatment steps such as preconditioning of donor plants, presoaking of explanted tissue, shaking during incubation, etc., were totally avoided. Protoplast from Lupinus luteus L. was also isolated from explants derived from in vitro raised young seedlings.
Most protoplast isolation protocols generally aim at higher yields, rapid recovery and regenerability of protoplasts so obtained. But all these objectives are seldom accomplished with any one method as there has been no demonstrated whole plant regeneration from protoplasts obtained by rapid preparations [7-9] and similarly, high-yielding protocols are rarely less simple procedures. Using the method described, we have overcome some of these problems. The protocol provides a simple and an easy to handle procedure that ensured satisfactory yields and quick recovery of viable protoplasts of this important tree.
Conclusion
Freshly obtained protoplasts are viable and morphologically regular. Here, isolation as a crucial step of protoplast utilization was comprehensively optimized for Oroxylum indicum, taking into account different concentrations and combinations of enzyme and incubation period, affecting the efficiency of the procedure.
Acknowledgements
Author is thankful to WOS-A, DST, New Delhi for financial assistance, and the Management of St. Aloysius (autonomous) College, Jabalpur for providing all necessary facilities.
References
- Davey MR, Anthony P, Power JB, Lowe K (2005) Plant protoplasts: statusand biotechnological perspectives. Biotechnol Adv 23: 131-171.
- Deka DC, Kumar V, Prasad C, Kumar K, Gogoi BJ, et al. (2013). OroxylumIndicum -a medicinal plant of north east India: an overview of its nutritional, remedial, and prophylactic properties. J Applied Pharmaceutical Science 3: 104-112.
- Kirtikar K, Basu B (2004) Indian Medicinal Plants. Oriental Enterprises, Dehradun. 4: 1105-1107.
- Gokhale M, Bansal YK (2009) Direct in vitro regeneration of a medicinal treeOroxylum indicum (L.) Vent. through tissue culture. African J Biotechnology 8: 3777-3781
- Gokhale M, Bansal YK (2010) Indirect organogenesis in Oroxylum indicum(L.) vent. Indian Forester 136: 804-811.
- Rao S, Prakash A (1995) A simple method for the isolation of plant protoplasts. J Biosci 20: 645-655.
- Moore Î’, Monson R, Ku μS, Edwards G (1988) Activities of principal photosynthetic and photorespiratory enzymes in leaf mesophyll and bundle sheath protoplasts from the C3-C4 intermediate Flavaria ramosissima. Plant & Cell Physiology 29: 999-1006.
- Thirumala DM, Vani T, Malla Reddy μ, Raghavendra A (1992) Rapid isolation of mesophyll and guard cell protoplasts from pea and maize leaves. Indian J Experimental Biology 30: 424-428.
- Thirumala DM, Vani T, Malla Reddy μ, Raghavendra A (1992) Rapid isolationof mesophyll and guard cell protoplasts from pea and maize leaves. Indian J Experimental Biology 30: 424-428.