Research Article
In Vitro Propagation and Conservation of a Medicinal Plant Rauvolfia serpentina (L.) Benth. Ex Kurz. From Similipal Biosphere Reserve
Mahapatra B*, Bhuyan JK and Hansdah BP*
Department of Botany, MSCB University, Takatpur, Baripada, Odisha. India
*Corresponding author: Mahapatra Bijayalaxmi, Department of Botany, MSCBD University, Takatpur, Baripada, Odisha. India E-mail Id: bijaylaxmimahapatra25@gmail.com
Bishnupriya Hansdah, Department of Botany, MSCB University, Takatpur, Baripada, Odisha. India
Bishnupriya Hansdah, Department of Botany, MSCB University, Takatpur, Baripada, Odisha. India
Copyright:©Mahapatra B, et al. 2024. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information:Submission: 19/01/2024; Accepted: 06/02/2024; Published: 09/02/2024
Abstract
Rauvolfia serpentina(L.) Benth. ex Kurz is commonly known as Sarpagandha is an important medicinal shrub of family Apocynaceae. This plant species is of great medicinal importance to treat cardiovascular diseases, hypertension, diabetes, malaria, cancer etc. The natural habitat of this species is
decreasing due to anthropogenic activities and rapidly eroding natural ecosystem. This plant has comparatively moderate propagation rate in nature. Present investigation is an effort to establish Rauvolfia serpentina for direct and indirect organogenesis from nodal and leaf explants. Leaf and shoot explants were
cultured on MS media supplemented with different concentrations of NAA, 2, 4-D, BAP and KIN were used either in singly or in combination. Among all the growth hormones 2, 4-D was the best for callus induction (94% in stem and 98% in leaf) and in combination 2, 4-D and BAP (84% in stem and 94% in leaf). Day of callus induction started from13th to 37th day. For direct regeneration from nodal segment, best growth of auxiliary shoot was obtained on MS medium supplemented with 1.5mg/l BAP and 0.5 mg/l NAA concentrations.
Keywords: Rauvolfia Serpentina; Callus; In Vitro Propagation
Introduction
The typical traditional systems of medicine for thousand years
have been in existence have formed from plants. The plant remains to
offer mankind with new medicines. At present, many well-established
herbal and plant medicine practices (Ayurvedic medicine in India) are
popular in many parts of the world. The World Health Organization
(WHO) reported that 80% of people in developing world use
medicinal plant for their primary health care [1]. The use of herbal
medicines is growing in developed countries, presently 25% of the UK
population use herbal medicine [2]. About 40% of compounds used
in pharmaceutical industry derived directly or indirectly from plants
[3] because the chemical synthesis of such compounds is either not
possible or economically not visible [4]. Therefore, a large number of
medicinal plant species are under threat of extinction because of their
overexploitation [5].
Rauvolfia serpentina (L.) Benth ex Kurz is commonly known, as
sarpagandha is an important medicinal shrub of family Apocynaceae
[6] which grow up to 60m of height. The total numbers of 80 species
are included in this genus. It is also known as chandrabhaga,
snakeroot plant, chhootachand, Chandrika, Harkaya [7]. Its root
contains 0.15% reserpine and rescinnamine group of alkaloids. It
also contains a number of bioactive compounds including ajmaline,
deserpidine, rescinnnamine and yohimbine. The root of this plant
used as medicine for high blood pressure, insomnia, anxiety and
other disorders of central epilepsy [8].
The number of R. Serpentina species is slowly degrading in India
due to over-exploitation and random collection for commercial
purposes to meet the pharmaceutical industry coupled with limited
cultivation [9-11]. Another reason for degradation of this plant is
poor seed germination. This plant germinates through vegetatively
by root cutting. The chemical reserpine is an alkaloid first isolated
from roots of R. serpentina and is used to treat hypertension [12,13].
Subsequently, other clinical investigators working in India confirmed
the effectiveness of R. serpentina for that purposes [14,15]. In short-term
study, a significant decrease in systolic as well as diastolic blood
pressure of patients was observed [16].The pectic polysaccharide
named rauwolfian RS was obtained from the dried callus of R.
serpentina by extraction with 0.7% aqueous ammonium oxalate and
it was found to possess some anti-inflammatory effect [17]. Therefore,
major steps have been taken to conserve this medicinal plant.
Ex-situ conservation through plant tissue culture is very successful
for mass propagation of several plant species. Micro propagation
can be considered as an important tool for the production of higher
quality based plant-based medicines. Regarding this view there is an
urgent need to apply in vitro culture methods for micro propagation
and conservation of this valuable plant. In vitro regeneration of
Rauvolfia has been reported by many authors [18,19,9,7]. With
this insight there is an urgent need to apply in vitro culture methods
for micro propagation and conservation of this valuable plant. The
present study was undertaken to develop a more efficient protocol for
rapid in vitro multiplication of R. serpentina.
Materials and Methods
Collection and sterilization of explants:
R.serpentina was collected from Similipal Biosphere Reserve
and was planted in departmental garden of Dept. of Botany,
MSCBU, Takatpur, Baripada. Young stem (nodal) and leaves of R.
serpentina were taken as explants in the present study. The explants
were thoroughly washed with running tap water followed by double
distilled water and 70% ethanol for 30 seconds. These were also
treated with labolene solution for 5 minutes under Laminar airflow.
Subsequently the stems and leaves were finally sterilized with 1%
of sodium hypochlorite solution (NaOCl) for 10 minutes and then
washed with sterile distilled water for 4-5 times. Stems and leaves
were then dried using sterile tissue paper and excised into segment
and inoculated onto prepared medium.Preparation and sterilization of media:
For in vitro culture of R. Serpentina MS medium [20] was taken.
To prepare 1 litre medium requisite amount of sucrose (30 g/l) and
agar (8g/l) were added. Various growth regulators like auxins or
cytokinins were also added according to required amount. The pH of
the medium was adjusted to 5.6 to 5.8 and the media were autoclaved
at 15 Ibs/inch2 for 20 minutes at 121°C for proper sterilizationCallusing:
For callus induction, juvenile stem (nodal) and leaf cut about
5 mm in length were aseptically prepared and were implanted
vertically on MS medium prepared with specific concentrations of
hormones. Culture of stem and leaf explants were initially incubated
under darkness in a culture chamber at 25°C for callus induction.
Subsequently, explants were incubated under a 16/8-h (light/ dark)
photoperiod with cool-white fluorescent lighting at an intensity of 60
μE·m–2·s–1 intensity at a constant temperature of 25°C ± 2°C.Direct regeneration of Shoot:
For direct shoot regeneration, the nodal segment was cut into small
pieces and each piece of nodal segment was transferred to MS media
having growth hormones in similar composition and concentration
as for shoot regeneration and light treatments were same as for callus
induction. After 3-4 weeks, direct shoot regeneration occurs from
nodal segment. After 5-6 weeks of old shoots were cut into 3.5 cm in
length and cultured on MS media having same growth hormones in
similar composition, concentration and incubation as for shooting
and after 75 days complete plantlets were formed.Results
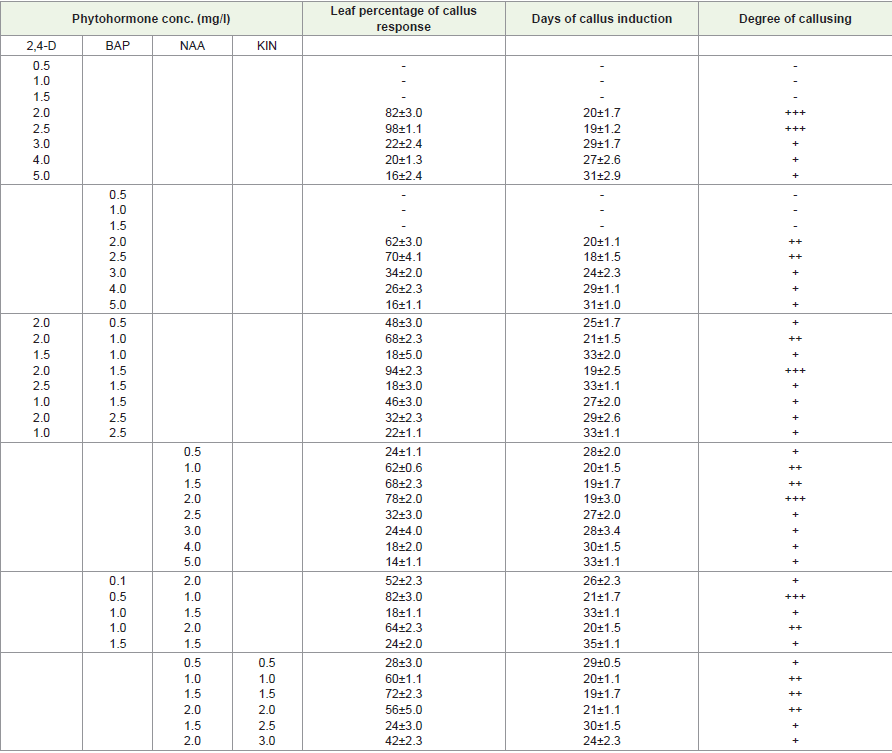
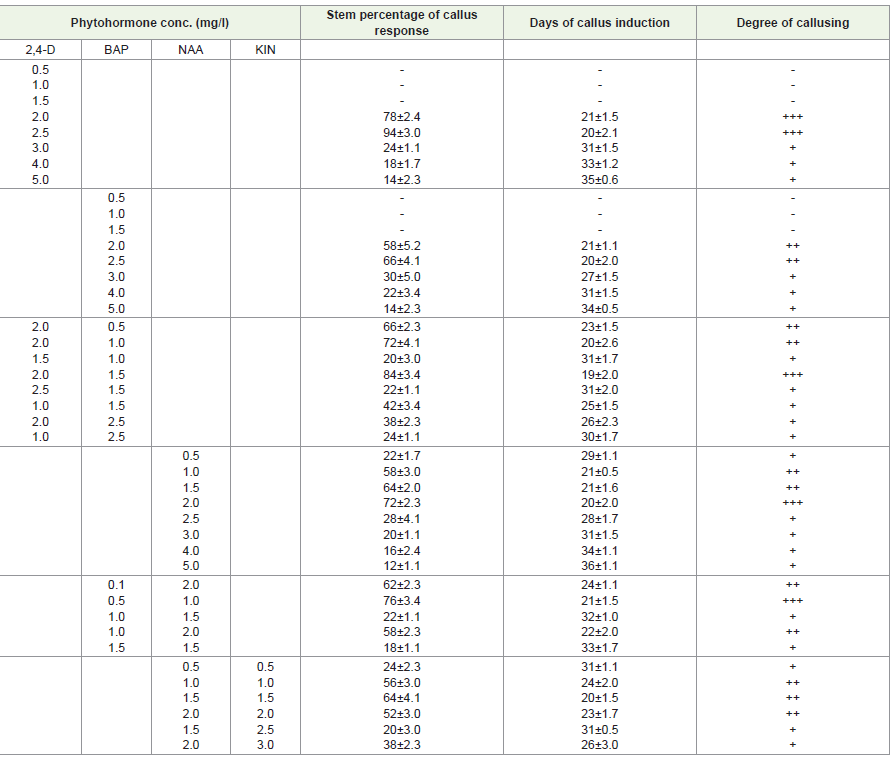
Effects of different concentrations of auxin and cytokinin singly on callus induction:
MS media supplemented with different concentrations of (Figure
1) 4-Dichlorophenoxyacetic acid (Figure 1), (Figure1-D) showed
stimulatory effects on callus induction. Maximum callusing response
(94% in stem and 98% in leaf) was noted at 2.5mg/l. At 10mg/l no
callusing or growth was observed.MS media supplemented with different concentrations of
1-Naphthaleneacetic acid (NAA) showed stimulatory effects on callus
induction. Maximum callusing response (72% in stem and 78% in
leaf) was recorded at 2 mg/l of NAA. At 0.5mg/l the callusing response
was recorded less and it increased up to 2mg/l. At 2.5mg/l onward
callusing response was reduced and found minimum at 5mg/l. At
10mg/l no callusing or growth was observed. It was observed that the
higher concentration of NAA in media had an inhibitory effect on
callus proliferation.
MS media supplemented with different concentrations of 6-
Benzyl aminopurine (BAP) showed stimulatory effects on callus
induction. Maximum callusing response (66% in stem and 70% in
leaf) was noted at 2.5mg/l of BAP. Lower concentrations of BAP
(0.5mg/l to 1.5mg/l) were unable to induce callusing and higher
concentrations of BAP (10 mg/l) in media had an inhibitory effect on
callus induction.
But MS medium supplemented with concentrations of 0.5 mg/l to
10 mg/l of Kinetin (KIN) callus formation was not observed on stem
and leaf explants.
Effects of different concentrations and combinations of growth hormones on leaf and stem callus induction:
MS media supplemented with different concentrations of (Figure
1), (Figure1-D)-Dichlorophenoxyacetic acid (Figure 1), (Figure1-D)
and 6-benzylaminopurine (BAP) showed stimulatory effects on
callus induction. Maximum callusing response (84% in stem and
94% in leaf) was recorded at 1mg/l of BAP and 2mg/l of (Figure 1),
(Figure1-D). At 3mg/l of BAP and 1 mg/l of (Figure 1), (Figure1-D)
swelling of callus was observed. At 5mg/l to 10mg/l of BAP and
(Figure 1), (Figure1-D)no callusing or growth was observed.MS media supplemented with different concentrations of
1-Naphthaleneacetic acid (NAA) and 6-Benzylaminopurine (BAP)
showed stimulatory effects on callus induction. Maximum callusing
response (76% in stem and 82% in leaf) was recorded at 0.5 mg/l of
Figure 1:A-I: Micropropagation of Rauvolfia Serpentina; (A-C): Callus Formation from Leaf Explants; (D-F): Callus Formation from Nodal Explants; (G-I): Multiple Shoot Formation from Nodal Explants.
BAP and 1 mg/l of NAA. At 2.5 mg/l to 10 mg/l of BAP and NAA no
callusing or growth was observed.
MS media supplemented with different concentrations of
1-naphthaleneacetic acid (NAA) and KIN showed stimulatory effects
on callus induction. Maximum callusing response (64% in stem and
72% in leaf) was recorded at KIN 1 mg/l and NAA 1.5 mg/l. At 2.5
mg/l to 10mg/l of KIN and NAA no callusing or growth was observed.
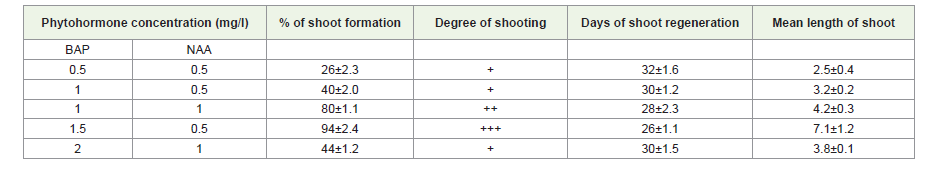
Direct shoot regeneration from nodal segment:
The plant is vegetatively propagated by root cutting because
of seed are mostly non-viable due to abortive embryos and low
germination percentage. The in vitro multiplication of R. serpentina
shoots through nodal segment is the most commercially viable
means of micro propagation. It is also useful for increasing number
of shoots, which originally differentiated in vitro. The present study
deals with the in vitro propagation for direct regeneration from nodal
segments R.serpentina. Best growth of axillary shoots were obtained
from MS media supplemented with 1.5 mg/l BAP and 0.5 mg/l NAA
concentrations were applied to induce direct regeneration followed
by BAP 1 mg/l and NAA 1 mg/l in nodal explants.Discussion
In the present work, two explants leaf and nodal stem were used
in which leaf explants were found best for callus induction than
stem, which is in accordance with the earlier findings [21]. In vitro
regeneration of R. serpentina has been reported by many authors [22-26]. For the preparation of media standard procedure was followed
[27]. MS media without any growth hormone was unable to induce
callus [27]. The auxins facilitate cell elongation and root initiation
while the cytokinins induce cell division and differentiation. Among
all the growth hormones, (Figure 1), (Figure1-D) was the best for
callus induction in leaf explants (Table-1) (Figure-1C). Present
results are also in accordance with the result reported by [23] Mitra
and Kaul in 1964. (Figure 1), (Figure1-D)is an elective herbicide with
auxin activity. The herbicide is especially designed to control broad
leaf weeds (dicotyledons) in cereal crop fields. It is generally accepted
that (Figure 1), (Figure1-D)is an auxin-like herbicide, because at low
concentration it has growth promoting properties. The first herbicide
reported to improve growth and yield of crops at sub toxic level was
[Figure 1], (Figure1-D) [28]. (Figure1), (Figure1-D)is usually used
with cytokinins for callus induction. The induction of callogenesis
was also conformed in Kalanchoe blossfeldiana pollen and Digitalis
lanataEhrh [29,30]. [Figure 2], (Figure1-D) is an auxin and plays a primary role in cell elongation and root initiation [31]. The response
of explants in this study might be due to the auxin- cytokinin
balance derived from exogenous auxin [Figure 1], (Figure1-D) and
endogenous auxin cytokinin in plant cells.
The cytokinins and auxins are important in in-vitro culture
as the later are concerned with root formation, and the former are
mainly required for shoot formation and growth of buds [32].These
growth regulators are required in combination as it is always the
manipulation and variation in auxins and cytokinins level that can
successfully change the growth behaviour of plant cultures [34].
Cytokinins such as BAP and kinetin are known to reduce the apical
meristem dominance and induce both auxiliary and adventitious
shoot formation [35]. The application of higher concentration of BAP
inhibits elongation of adventitious meristems and the conversion into
complete plant. Auxins and other growth regulators play important
roles in growth and differentiation of cultured cell and tissue [36].In
our present study we found that BAP alone is less effective in callus
formation than BAP in combination with auxins like 2,4-D and NAA
(Table-1), (Figure-1F). Similar results are reported by [37] Roja and
Heble in 1996 where MS media supplemented with 2, 4-D and BAP
was found best for callus induction. Some workers have reported
that BAPin combination with other auxins like IAA and IBA shows
positive results in callus formation of R. Serpentina [9,38] which is
different from our results.
NAA comes under auxin family plays an important role in rooting
agent. NAA is too toxic at higher concentration to plants but at lower
concentration, it shows growth and development of plant tissue [39].
In our present study, NAA in combination with cytokinin like BAP
shows better results in callus regeneration than alone [Table 1],
[Table 2]. In the present work phytohormone named Kinetin (KIN)
alone could not induce callus [40] . In further experiments Kinetin
was supplemented to the MS media in combination with auxins (2,4-D and NAA).
It was observed that KIN had enhanced callus growth
in presence of auxins. Day of callus induction started from 17th to 37th
day [41]. This variation observed in the present investigation may be
attributed due to the difference in culture conditions and the age of
explants.
Table 3:Direct shoot regeneration from stem nodal explants on MS medium under the influence of different concentrations of phytohormones
The in vitro multiplication of Rauvolfia serpentina shoots
through nodal segment is the most commercially viable means of
micro-propagation. It is also useful for increasing number of shoots,
which originally differentiated in vitro. Best growth of axillary
shoots was obtained on MS supplemented with concentration and
combination of phytohormones containing 1.0 mg/l BAP and 0.1
mg/l NAA[Table 3], [Figure 1]. Thus, the propagation of plants from
nodal segments has proved to be the most generally applicable and
reliable method of in vitro propagation in Rauvolfia serpentina, as
the regeneration of the plant is very difficult from seeds and other
sources. The seeds are mostly non-viable due to abortive embryos
[42,43].
The present work describes a reproducible and efficient protocol
for propagation of R. serpentina as an important medicinal plant
species from leaf and stem explants. Propagation from nodal explants
through direct organogenesis removes the need for an intervening
callus phase and avoids the use of mercuric chloride, thus assuring
the species effective establishment and multiplication irrespective
of seasonal constraints. This micro propagation technique could
support the conservation of this valuable plant species to protect
it from indiscriminate exploitation. It could work as useful tool to
increase biomass and yield of pharmaceutically important alkaloids
and photochemical accumulated in Rauvolfia serpentina.
Acknowledgement
Authors are thankful to authorities of P.G Department of Botany,
MSCBD University. Thanks are also due to Dr. Kishore K. Mandal,
Dr. Truptirekha Kar and Mr Sudhir K. Behera for assistance in
experimental work.
References
10. Gupta R (1988) Genetic Resources of Medicinal Plants. Indian J. Plant Genet Resource, 1: 98-102.