Research Article
Determination of the Safe Dose of Aqueous Extract of Apium graveolens L. By Acute and Sub-Acute Toxicity Study
Kma L1*, Marbaniang C2, Tongbram Chanu M3 and NathSharan R4
Biochemistry Department, North Eastern Hill University (NEHU) Shillong, Meghalaya, India
*Corresponding author: Lakhon Kma, Biochemistry Department, North Eastern Hill University (NEHU) Shillong ,Meghalaya, India E-mail Id: lakhankma@gmail.com
Copyright: © Kma L, et al. 2023. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 29/03/2023; Accepted: 02/10/2023; Published: 07/10/2023
Abstract
Objective: The present study aims to determine the safe dose of the aqueous extract of Apium graveolens by acute and sub-acute oral toxicity study in BALB/c.
Methods: The acute toxicity effect of the plant extract was determined by a single oral administration at a dose (500, 1000, and 2000 mg/kg) and general behavior, adverse effects and mortality were observed for the first 4h up to 72h and compared to the normal group. The sub-acute toxicity effect of the plant the extract was determined by the oral administration at doses of 200 and 400 mg/kg for 28 days and their body weight, absolute organ weight; relative organ weight serum and liver biochemical parameters were evaluated and compared to the normal group.
Results: In acute toxicity, treatment with aqueous leaf extract of Apium graveolens showed no mortality, suggesting its median lethal dose (LD50) is >2000 mg/kg b.w. However, general signs of discomforts were observed at doses 500 and 1000mg/kg b.w. In sub-acute toxicity, no significant changes were observed in most of the tested parameters at a dose 200mg/kg b.w. However, major significant changes were observed in the group treated at doses 400mg/ kg b.w such as body weight, organ weight, ROW as well as in biochemical parameters, in particular SGPT, SGOT, Uric acid, ALP, LDH, hepatic oxidative stress markers and fasting lipid profile when compared to control group.
Conclusion: For further studies that required prolonged exposure of more than 28 days, we suggest a dose ≤200mg/kg b.w of Apium graveolens aqueous extract.
Methods: The acute toxicity effect of the plant extract was determined by a single oral administration at a dose (500, 1000, and 2000 mg/kg) and general behavior, adverse effects and mortality were observed for the first 4h up to 72h and compared to the normal group. The sub-acute toxicity effect of the plant the extract was determined by the oral administration at doses of 200 and 400 mg/kg for 28 days and their body weight, absolute organ weight; relative organ weight serum and liver biochemical parameters were evaluated and compared to the normal group.
Results: In acute toxicity, treatment with aqueous leaf extract of Apium graveolens showed no mortality, suggesting its median lethal dose (LD50) is >2000 mg/kg b.w. However, general signs of discomforts were observed at doses 500 and 1000mg/kg b.w. In sub-acute toxicity, no significant changes were observed in most of the tested parameters at a dose 200mg/kg b.w. However, major significant changes were observed in the group treated at doses 400mg/ kg b.w such as body weight, organ weight, ROW as well as in biochemical parameters, in particular SGPT, SGOT, Uric acid, ALP, LDH, hepatic oxidative stress markers and fasting lipid profile when compared to control group.
Conclusion: For further studies that required prolonged exposure of more than 28 days, we suggest a dose ≤200mg/kg b.w of Apium graveolens aqueous extract.
Keywords:Apium graveolens; Aqueous Extract; Toxicity Study; Behavioral Changes; Serum and Tissue Analysis
Introduction
Traditional medical practices have relied on plants for curing
human illness for ages [1]. This is because polyphenols (including
flavonoids, phenylpropanoids, phenolic acids, tannins, etc.) play
a significant role in the therapeutic effects of plants [2]. The high
expense of Western pharmaceuticals and healthcare [3] means that
plants continue to make a vital contribution to health care despite
the great gains observed in modern medicine in recent decades.
Compared to their synthetic counterparts, medicines made from
plant sources have less negative effects [4]. Studies of medicinal
plants (raw or extracted compounds derived from plants) for a wide
range of ailments, including cancer, infectious disorders, diabetes,
and atherosclerosis, have been conducted over the course of centuries
[5]. However, several severe adverse outcomes can be caused by
misuse these herbs, including overdose, over duration, tolerance,
dependency, and addiction [6]. The plant extract’s components may
interact with the target receptor in a number of ways, some of which
are harmful. Another way that the plant extract’s components can
trigger signaling pathways is by random receptor binding [7]. Even
seemingly harmless compounds can produce poisonous byproducts
when broken down in the liver or kidneys [8] or trigger unexpected
reactions in some people [9]. If not eliminated from the body,
harmful substances or metabolites build up and can cause disease
through interactions with macromolecules like DNA or proteins,
forming DNA adducts or proteins adducts [10,11]. Because of this,
pharmacological studies of numerous medicinal plants are required.
Apium graveolens, more often known as celery, has been utilized
for centuries in both Western and Eastern medicine [12,13]. Its
medical value and effectiveness have been discussed elsewhere [5,14,15]. The stem, the leaves, and the roots of celery all have fragrant
properties. Celery’s therapeutic benefits come from the essential oil
and flavonoids it contains [15]. The solvent used to extract bioactive
phytochemical components from celery is crucial. Aqueous extract
had a larger concentration of phenolic and flavonoid compounds, as
well as superior antioxidant and in-vitro anti-inflammatory effects
[16]. Since little is known about the safety of Apium graveolens
aqueous extract, the current study sought to fill this knowledge gap
by conducting acute and sub-acute toxicity testing on BALB/c mice
to determine appropriate doses of the extract.
Materials and Methods
Chemicals:
All chemicals used were of analytical grade and were used
without further purification. H2O2, Bradford reagent, potassium
dichromate, DTNB (Ellman’s reagent), TCA, TBA, MDA, and HCl
were purchased from Sisco Research Laboratories (SRL), Mumbai,
India. Assay kits for serum SGOT, SGPT, Uric acid, ALP, Bilirubin,
LDH and Total Cholesterol (TC), Triglyceride (TG), High-density
lipoprotein cholesterol (HDL-C), were purchased from the Coral
clinical system, Pantnagar, India.Plant Material:
The fresh leaves of celery (Apium graveolens) were procured from
the local market at Shillong, Meghalaya, India. The leaves were washed
thoroughly with tap water and air-dried at room temperature, which
was then cut to small pieces and dried in an oven at 40 ºC for 3 days.
The dried leaves were then crushed to a fine powder using an electric
blender. The powdered sample was stored at 4 ºC in an airtight bottle.Preparation of Extract:
The powdered plant material was extracted with distilled water
at the ratio of 1:10 (powder/solvent) with intermittent shaking for
24 h. The extract was filtered by using muslin cloth followed by
Whatman No. 1 filter paper to obtain the filtrate. The filtrate was
then concentrated using a Rotary Evaporator and was lyophilized to
powder. The residue obtained was reconstituted in distilled water at
the appropriate concentrations and stored in the refrigerator at 4 °C.
The powder obtained was weighed to calculate the percentage yield
using the formula,
% Yield = (Weight of dry extract/ Weight taken for extraction × 100)Experimental Animals:
Swiss albino (BALB/c) adult mice weighing 20-30 g were obtained
from the Pasteur Institute, Shillong, Meghalaya, India. The animal
was grouped and housed in polyacrylic cages of 3 mice per cage
and maintained under standard laboratory conditions (temperature
25-28 ºC) with a 12 h light and 12 h dark cycle. They were allowed
free access to a standard dry pellet diet (Hindustan Lever, Kolkata,
India) and water ad libitum. The mice were acclimatized to laboratory
conditions for 7 days before the commencement of the experiment.
The experimental animals were handled according to Institutional
Animal Ethical Committee (IAEC) regulations of North-Eastern Hill
University, Shillong, Meghalaya, India.Acute Toxicity Study:
Using BALB/c mice (weighing 20-30 g), an aqueous extract of
Apium graveolens was tested for acute toxicity via oral administration
[17]. This investigation followed the guidelines established by the
Organization for Economic Co-operation and Development (OECD)
guideline 423. Before each trial, all the animals fasted on water alone
for a full day. Prior to dosing, the animals’ bodyweights were recorded
so that the appropriate amounts of medication could be given to each
group based on their individual weights. The first group was a placebo,
whereas the second through fourth groups were given oral gavages of
extract at 500, 1500, and 2000 mg/kg b.w. respectively. The mice were
fasted for just 4 hours after receiving the plant extract orally. For 72
hours, authors examined each animal for signs of distress or toxicity,
including changes in behavior, body weight, urine, food intake, water
intake, respiration, convulsion, tremor, temperature, constipation,
eye and skin color, etc., and mortality.Sub-acute study:
In the sub-acute study [18], the plant extract was administered
orally at doses of 200 and 400 mg/kg b.w. respectively for 28
consecutive days to the two groups and their body weight, organs
weight; relative organ weight, and hepatotoxicity were evaluated and
compared to the normal group. All the groups received a standard
pellet diet and water ad libitum.Collection of samples:
On the 29th day, after fasting for 18 h blood was collected from
retro-orbital sinus puncture under mild anesthesia and the animals
were sacrificed. Serum was separated by centrifugation at 3000 rpm
for 10 min at 4 ºC for estimation of biochemical parameters such as
aspartate transaminase (AST), alanine transaminase (ALT), LDH,
uric acid,alkaline phosphatase, and fasting lipid profile (FLP) were
determined for both control and extract treated groups. The liver,
brain, kidney, heart, and spleen were dissected, weighed each organ
on an electronic balance, and the relative organbody weight of both
test-treated groups was determined andcompared to the control
group. The relative organ weight (ROW) ofeach organ was calculated
as follows [19], and all vital organs were stored immediately at -80 ºC
for future analysis.
ROW = Absolute organ weight (g)/Mice body weight on sacrifice
day × 100Hepatotoxicity studies:
Hepatotoxicity was evaluated by measuring the level of AST, ALT,
LDH, uric acid, and tissue markers for oxidative stress and compared
with the normal group.Serum analysis:
The biochemical analysis was done on serum after centrifugation
of collected blood and the following parameters like aspartate
transaminase (AST), alanine transaminase (ALT), alkaline
phosphatase (ALP), LDH, gamma-glutamyltransferase, total and
direct bilirubin, uric acid, and fasting lipid profile (FLP) such as total
cholesterol, triglycerides, HDL-c level were determined for both
control and extract treated groups following kits instruction (Coral
Clinical System, Pantnagar, India). The level of serum VLDL and
LDL-c were estimated according to the Friedewald formula [20].Liver oxidative stress markers:
Liver homogenate (1 g) was prepared in a ratio of 1:10 (w/v) in
ice-cold 0.1 M TrisHCl buffer (pH 7.4) and homogenized by using a
Teflon homogenizer. The homogenate was centrifuged at 3000 rpm
for 20 min at 4 ºC and the supernatant was used for the estimation
of catalase (CAT) [21], reduced glutathione (GSH) [22], lipid
peroxidation product (Thiobarbituric Acid Reactive Substances –
TBARS) [23], and the total protein [24].Statistical Analysis:
Statistical analysis was performed using GRAPH PAD Prism
software package, Version 8.0. All the data wereexpressed as mean
± standard error mean (SEM).The comparison between groups was
evaluated utilizing an unpaired t-test. A less significant dose (p >>>
0.05), when compared to control, was selected for further studies.Results
The Percentage Yield of Extraction:
The percentage extract yield (W/V) calculated as (dry extract
weight/dry starting material weight) x 100 using aqueous as a solvent
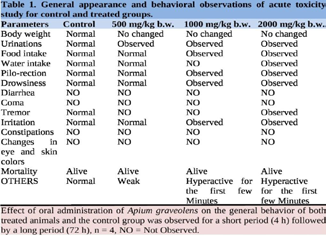
ranged from 26.6% to 32.2%.Acute Toxicity Study:
Oral dosage of 500, 1000, and 2000 mg/kg b.w. of aqueous extract
was used to test the acute toxic impact of the extract. There was no
mortality in any of the mice that were given the aqueous extract of
Apium graveolens, hence the LD50 was calculated to be >2000 mg/
kg. There were no fatalities reported, but indicators of discomfort,
including urination, increased food intake, piloerection, etc., were
seen with increasing doses, notably at 1000 and 2000 mg/kg, as shown
in Table 1. However, most of the widespread behaviors disappeared
at a dose of 500 mg/kg b.w. In this section, we provide the results of
the acute toxicity investigation, which involved the injection of an
aqueous extract (Table 1).Sub-acute toxicity study:
In the sub-acute toxicity study, doses <500 mg/kg b.w. were
selected based on the general behaviors observed from the acute
toxicity study. The acute toxic effect of aqueous extract was determined
by oral administration of the extract at doses of 200 and 400 mg/kg
b.w. for 28 consecutive days. All the tested group animals treated with
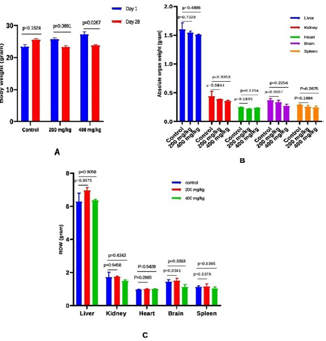
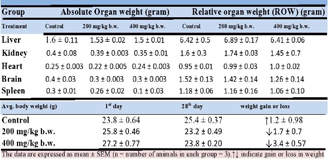
plant extract survived throughout the 28 days.Effect of Apium graveolens on body weight, average organ
weight, and relative organ weight (grams) in normal and celery
treated mice: Table 2 and Figure 1 show the final body, organ,
and relative organ weight changes after the treatment period. On
comparing day 1 and day 28 weights in the control (p=0.1624) and
200 mg/kg (p=0.0691) groups, there were no statistically significant
differences in body weight changes. Weight differences at 400 mg/kg
were statistically significant (p=0.0267). At the end of the treatment
period, it was also noted that the treated groups had lost weight while
the control group had gained weight. Table 2 shows that weight loss
was not statistically significant in the 200 mg/kg group but was at the
Table 1: General appearance and behavioral observations of acute toxicity
study for control and treated groups.
Figure 1: Effect of Apium graveolens on body weight (A), absolute organ
weight (B), and relative organ weight (C) (grams) in normal and celery
treated mice. All the data were expressed as mean ±SEM and N = 3. The
comparisons were analyzed by unpaired t-test (treated vs. control). Less
significant doses with p>>>0.05 was considered as a safe dose (p-value of
200 vs. 400 mg/kg b.w.).
Table 2: Effect of aqueous extract of Apium graveolens on body weight,
organ weight and relative organ weight (gram) in normal and treated mice.
400 mg/kg dose. Changes in absolute organ weight (g) and relative
organ weight (Absolute organ weight (g)/Mice body weight on
sacrifice day 100) were also not significantly different between the
control and treatment groups.
Hepatotoxicity studies: The ability of the plant extract to induce liver injury at two different doses (200 and 400 mg/kg) was evaluated
in both serum and liver.
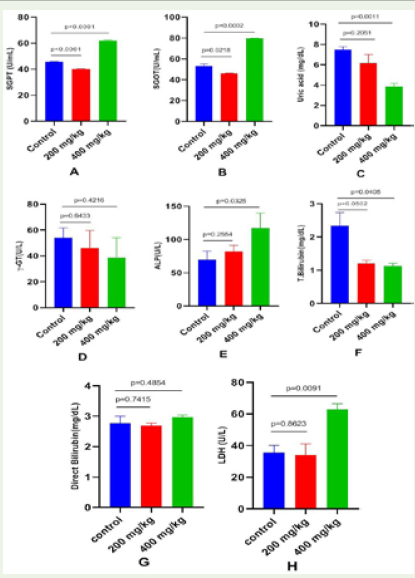
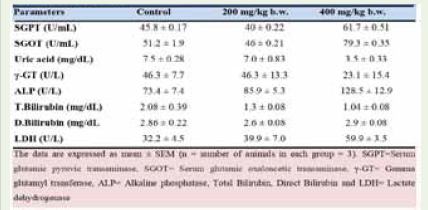
Effect of Apium graveolens on serum enzymes in normal and celery treated mice: Serum liver enzyme indicators were affected
differently by 28 days of oral administration of Apium graveolens
aqueous extract at 200 mg/kg and 400 mg/kg b.w. Summarized here
are the findings from a battery of biochemical analyses performed on
both the treatment and control groups (Table 3 and Figure 2). In groups
given extract at 200 mg/kg b.w., serum levels of uric acid (p=0.2052),
gamma-glutamyltransferase (p=0.6433), alkaline phosphatase
(p=0.2555), total bilirubin (p=0.052), direct bilirubin (p=0.7415), and
lactate dehydrogenase (p=0.08623) were not significantly reduced.
There was a statistically significant rise in uric acid (p=0.0011), alkaline
phosphatase (p=0.0325), total bilirubin (p=0.0405), direct bilirubin
(p=0.7415), and lactate dehydrogenase (p=0.0091) in the treatment
group compared to the control group. When comparing plant-treated
mice to control mice, SGPT levels were significantly lower (p>0.0001)
and SGOT levels were significantly higher (p=0.0218) at a dose of 200
mg/kg, while SGPT levels were significantly lower (p>0.0001) and
SGOT levels were significantly higher (p=0.0002) at a dose of 400 mg/
kg. In all examined parameters, however, the p-value indicated that
the changes at 400 mg/kg b.w. were statistically significant compared
to those at 200 mg/kg b.w (Figure 2).
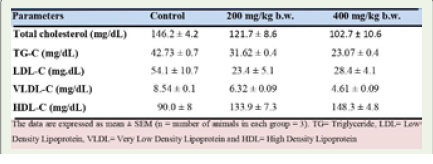
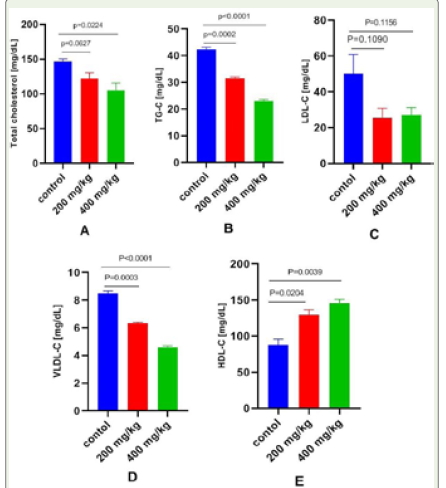
Effect of Apium graveolens on serum lipid in normal and celery treated mice: The results of the serum fasting lipid profile
are summarized in (Table 4 and Figure 3). Oral administration
of an aqueous extract of Apium graveolensm for 28 days at a dose
of 200 and 400 mg/kg resulted in a significant decrease in serum
lipid in the treated groups in comparison with the control. These
include cholesterol (p=0.0627) (p=0.0224), triglycerides (p=0.0002)
(p≤0.0001), LDL-C (p=0.1090) (p=0.1156) and VLDL-C (p=0.0003)
(p≤0.0001). There was also a significant increase in HDL-C level
(p=0.0204) (p=0.0039) when compared to control mice. However,
similar to serum enzyme (Figure 2), the effects were more pronounced
at the dose of 400 mg/kg, than at 200 mg/kg b.w. (Figure 3).
Figure 2: Effects of Apium graveolens on serum enzyme levels of control and
treated mice (A) Aspartate aminotransferase, (B) Alanine aminotransferase,
(C) Lactate dehydrogenase (D). All the data were expressed as mean ±SEM
and N = 3. The comparisons were analyzed by unpaired t-test (treated vs.
control). Less significant doses with p>>>0.05 was considered as a safe
dose (p-value 200 vs. 400 mg/kg b.w.).
Table 3: Effect of aqueous extract of Apium graveolens on body weight,
organ weight and relative organ weight (gram) in normal and treated mice.
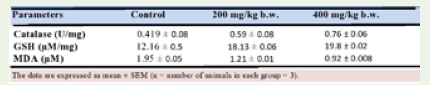
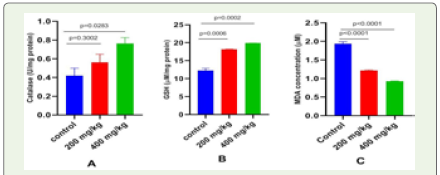
Effect of Apium graveolens on oxidative stress in normal and celery treated mice: (Table 5) and (Figure 4) describe the effect
on antioxidant enzymes after 28 days of oral treatment of Apium
graveolens aqueous extract at 200 mg/kg and 400 mg/kg b.w. By
comparing mice in the extract-treated group at 200 and 400 mg/kg to
mice in the control group, there was a substantial increase in catalase
activity (p=0.3002), (p=0.0283), and the level of GSH (p=0.0006),
Table 4: Effect of aqueous extract of Apium graveolens on fasting lipid
profile in normal and treated mice.
Table 5: Effect of aqueous extract of Apium graveolens on oxidative stress
parameters in normal and treated mice.
Figure 3: Effect of Apium graveolens on lipid profile in normal and celery
treated mice. A = Total cholesterol, B = Triglycerides, C = Low-Density
Lipoprotein, D = Very Low-Density Lipoprotein and E = High-Density
Lipoprotein. All the data were expressed as mean ±SEM and N = 3. The
comparisons were analyzed by unpaired t-test (treated vs. control). Less
significant doses with p>>>0.05 was considered as a safe dose (p-value
200 vs. 400g/kg b.w.).
(p=0.0002). Aqueous extract of Apium graveolens supplementation at
doses of 200 and 400 mg/kg b.w. also reduced MDA levels (p>0.0001).
Inhibition of lipid peroxidation, as shown by a dramatic drop in
MDA content (an end product of lipid peroxidation), is a hallmark of
the plant extract studied here (Figure 4).
Figure 4: Effect of Apium graveolens on oxidative stress markers (A =
Catalase, B = GSH, and C = MDA) in control and treated mice. All the data
were expressed as mean ±SEM and N = 3. The comparisons were analyzed
by unpaired t-test (treated vs. control). Less significant doses with p>>>0.05
was considered as a safe dose (p-value 200 vs. 400 mg/kg b.w.).
Discussion
Medicines and extracts from plants have been used for centuries
to treat human illness, and they are generally accepted as safe because
of their natural origin [25]. The presence of specific phytochemical
substances that cause a measurable physiological response in humans
is a major factor in the therapeutic usefulness of plant extracts [26].
However, not all phytochemicals that plants generate are non-toxic to
humans [27]. An acute toxicity test [28] is one method for determining a substance’s potential risk to humans and the environment.
Predicting the anticipated clinical symptoms requires toxicology
data. It provides a dosing range that can be utilized in potential future
experiments. An aqueous extract of Apium graveolens (celery) was
shown to have the highest extraction yield, antioxidant, and in-vitro
anti-inflammatory activity in our previous study [16]. Hence, we did
this study to find out how much of the plant’s aqueous extract is safe
to use in future research.
Since all experimental groups survived after receiving 500, 1000,
and 2000 mg/kg of Apium graveolens aqueous extract, it follows
that the median fatal dose (LD50) is greater than 2000 mg/kg. Any
substance having an LD50 > 1000 mg/kg b.w. is probably safe to use
[29]. There were no recorded fatalities, but significant discomfort
was seen in mice, notably at 1000 and 2000 mg/kg b.w. (Table 1). A
dose of ≤500 mg/kg of the plant extract would be adequate for longterm
treatment in the mouse model, as the majority of the measured
parameters of discomforts were not seen in treated mice. This is why
two dosages of Apium graveolens aqueous extract (200 and 400 mg/
kg b.w.) were chosen for a 28-day sub-acute toxicity investigation.
The toxicity study compared the animals’ initial and final weights
after they were given either of the two doses of the extract for 28
days. There were no statistically significant differences between the
control group and the 200 mg/kg treatment group in terms of weight
loss or gain (p > 0.05). At 400 mg/kg, however, as shown in (Table 2)
and (Figure 1), there were statistically significant variations in body
weight. Certain phytochemicals found in the aqueous extract at high
concentration may have contributed to the small weight loss (group
treated at 200 mg/kg) and the large weight loss (group treated at
400 mg/kg) compared to the corresponding controls. Toxic effects
in mice were detected by changes in body weight, suggesting that
these phytochemicals may have disrupted nutrition absorption in
the stomach and other metabolic processes. Inhibition of pancreatic
lipase and alpha-amylase is one way by which phytochemicals cause
weight loss [30]. Phytochemicals can also affect weight loss through
a mix of central and peripheral processes. Thus, 200 mg/kg remains
the optimal dose for chronic exposure in light of the statistical
discrepancies. Important organs like the liver, kidneys, heart, and
spleen demonstrate no effect from the extract at any dose throughout
therapy, with no statistically significant changes between treated and
control groups (P > 0.05).
Serum biochemical parameter estimates showed no significant
differences (P > 0.05) between groups treated with doses of 200 mg/
kg b.w. of the extract and the control group for uric acid, gammaglutamyltransferase,
ALP, Total Bilirubin, Direct Bilirubin, and
LDH. Mice given a plant extract at a concentration of 400 mg/kg had
considerably higher levels of ALP and LDH than control mice. The
hepato-protective effect of the extract was also demonstrated by a
significant reduction in SGOT and SGPT levels in the group given
200 mg/kg. However, as demonstrated in table 3 and figure 2, the
serum SGOT and SGPT levels considerably increase with increasing
dose (400 mg/kg b.w.). A possible cause for an increased serum
level of liver markers in the group given 400 mg/kg is the presence
of certain phytochemical compounds in the aqueous extract, which
may have toxic potential on the liver with increasing dose or can be
metabolized to other compounds, some of which may or may not be
hepatotoxic to the mice [31]. In the group given 200 mg/kg, there
were no detectable toxicological changes in any of the aforementioned
measures, suggesting that the plant extract can be safely used at doses
below 200 mg/kg body weight.
Fasting serum lipid profiles were significantly improved in the
treatment groups after 28 days of oral administration of an aqueous
extract of Apiumgraveolen sat 200 and 400 mg/kg body weight.
Increased levels of cholesterol, triglycerides, low-density lipoprotein
(LDL), and very low-density lipoprotein (VLDL) in the blood are
known to be key risk factors for the development of cardiovascular
disease [32]. HDL-C is referred to as “good” cholesterol since it is
responsible for transporting cholesterol from the bloodstream to
the liver, where it is either processed further or removed. Likewise,
HDL-C prevents LDL-C uptake by competing for LDL receptor
sites on arterial smooth muscle cells [33]. Hence, the better for the
body’s cells a higher serum HDL-C. Table 4 and Figure 3 show that
after treatment, both groups experienced a considerable reduction in
total cholesterol, triglyceride, very low density lipoprotein, and low
density lipoprotein levels, while HDL-C levels increased significantly
(p> 0.05). In conclusion, serum TC, TG, LDL-C, and VLDL-C
levels might be lowered by administration of an aqueous extract of
Apium graveolens. Clinical problems may arise if patients treated at
a dose of 400 mg/kg experience severe weight loss and a reduction
in serum lipids beyond the normal range (Table 2 and Figure 1).
Apium graveolens leaf extract has shown promise as a treatment for
hyperlipidemia due to its capacity to dramatically lower body weight,
improve lipid profile, and increase HDL-C; despite this promise, the
most effective dose was shown to be 200 mg/kg b.w.
Both endogenous and external factors contribute to the
production of reactive oxygen species (ROS) in the biological
system. Nevertheless, oxidative stress, which can contribute to the
development of various illnesses [34], can occur when production
exceeds the cell’s intrinsic antioxidant capability. Direct ROS
scavenging is just one of the many roles that biological antioxidants
can play. Antioxidant enzymes like catalase, glutathione (GSH), etc.
[35] are examples of such molecules. Catalase’s principal function is
the catalytic decomposition of hydrogen peroxide, which prevents
the formation of reactive hydroxyl radicals [36,37]. GSH helps
repair ROS-induced cellular damage [38] and acts as a free radical
scavenger. GSH is converted to GSSG during the removal of hydrogen
peroxide, and GSSG is converted back to GSH by GSH-reductase
[39]. Malondialdehyde (MDA) is a byproduct of lipid peroxidation
(LPO) [40]. Several MDA-like compounds are produced during LPO,
which has been linked to multiple disorders [41]. Using increasing
doses of Apium graveolens aqueous extract, we found that catalase
and GSH activities rose significantly (p>0.05). According to table 5
and figure 4, our results also shown that hepatic MDA concentration
was dramatically decreased by aqueous extract of the plant in a dosedependent
manner. Our findings demonstrated the plant extract’s
anti-oxidative impact against oxygen free radicals by increasing
the expression of the biological antioxidant defense system and its
ability to inhibit LPO by lowering MDA content in the treated group.
However, taking into account all parameters from the acute to the
sub-acute studies, a dose of 200 mg/kg b.w. appears to be favorable for
long-term treatment. The group treated with 400 mg/kg b.w. showed
highly significant elevation of the biological antioxidant enzymes and
LPO inhibition in the oxidative stress study.
Conclusion
Aqueous leaf extract of Apium graveolens is not likely to produce
any severe toxic effects. Its median lethal dose (LD50) of greater than
2000 mg/kg body weight justifies its safety. However, prolonged
oral administration at doses ≥ 400 mg/kg b.w. may cause significant
changes in body weight, organ weight, ROW as well as in biochemical
parameters, in particular SGPT, SGOT, uric acid, ALP, LDH, hepatic
oxidative stress markers and fasting lipid profile beyond the normal
level. Therefore, for further studies that required prolonged exposure
of more than 28 days, we suggest a dose ≤ 200 mg/kg b.w. of Apium
graveolens extract.