Review Article
Nanotechnology as an Anticancer Approach
Londonkar R and Kesaralikar M*
Department of Biotechnology, Gulbarga University, India
*Corresponding author: Kesaralikar M, Department of Biotechnology, Gulbarga University Gulbarg, Gulbarga Karnataka 585102, India; Email: maithilee1988@gmail.com
Copyright: © 2020 Londonkar R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information: Submission: 30/04/2020; Accepted: 28/05/2020; Published: 02/06/2020
Introduction
The WHO has recently defined traditional medicine (including
herbal drugs) as comprising therapeutic practices that have been in
existence, often for hundreds of years, before the development and
spread of modern medicine and are still in use today. Traditional
medicine is the synthesis of therapeutic experience of generations of
practicing physicians of indigenous system of medicine. Traditional
preparations comprise medicinal plants, minerals and organic matter
etc. Herbal drugs constitute only those traditional medicines which
primarily use medicinal plant preparations for therapy. The earliest
recorded evidence of their use in Indian, Chinese, Egyptian, Greek,
Roman and Syrian texts dates back to about 5000 years. The classical
Indian texts include Rigveda, Atharvaveda, Charak Samhita and
Sushruta Samhita. The junction of the rich knowledge from different
traditional systems of medicine can lead to new avenues in herbal
drug discovery process [1]. The combination of herbal medicine with
the nanotechnology is nowadays has becoming a great strategy for
delivering the drug. Using modern techniques, Herbal drug could
provide novel molecular probes. It is now possible to explore the
mechanism of action of herbal drugs in terms of current concept of
molecular pharmacology.
Nanotechnology is defined as applied science and technology
which aims to develop devices and dosage forms in the range of 1 to
100 nm. The applications of nanotechnology for treatment, diagnosis,
monitoring, and control of biological systems have recently been
referred to as nanomedicine. Nanosystems output is the active
constituent at a sufficient concentration during the entire treatment
period, directing it to the desired site of action. Conventional
treatments fail to complete these requirements. The purpose of this
study is to review nanotechnology-based drug delivery systems. Most
of the biologically active constituents of extracts, such as flavonoids, tannins, and terpenoids, are highly water-soluble, but demonstrate
a low absorption, because they are unable to cross lipid membranes,
have high molecular sizes, and demonstrate poor absorption, resulting
in loss of bioavailability and efficacy. Some studies have shown that
herbal medicines have good activity in assays in vitro, which are not
reproducible in experiments in vivo [2].
Furthermore, some essential elements like (microsphere and
liposomes) properties. E.g. Although the majority of this work
includes the use of microspheres, liposomes and gels are limited to
the delivery of macromolecules (e.g., insulin and growth hormone)
[3]. Several nanotechnological strategies, such as polymeric
nanoparticles, solid lipid nanoparticles (SLNs), liquid crystal (LC)
systems, precursors systems for liquid crystals (PSLCs), liposomes,
and micro emulsions, have attempted to break this barrier; they
allow substances with different properties to be used in the same
formulation, and may even change a substance’s properties and
behavior in a biological environment. These technological discoveries
have revolutionized process of drug delivery. The new drug delivery
systems have the ability not only to increase the effectiveness of active
components, but also to reintroduce other components that could
help in the drug delivery process and ultimately the enhancement
of drug efficacy as well as its effectiveness. Moreover, the ability
to improve new substances, such as by increasing selectivity and
efficacy, protecting against thermal- or photo-degradation, reducing
side effects, and controlling the release of active constituents, before
they are introduced to the market or used therapeutically, makes this
approach even more attractive.
Strategies of Nanotechnology as Novel Drug Delivery System (Ndds):
Nanoparticles (NPs) are the new identified tools by which drugs
can be delivered into tumor cells with minimum drug leakage into normal cells. Drug delivery system fetched a NDDS (Novel Drug
Delivery Systems), a novel approach to overcome the drawbacks of
the traditional drug delivery systems [4]. Nano-sized delivery system
was selected because of the following reasons:• They appear to be able to deliver high concentrations of
drugs to disease sites because of their unique size and high
loading capacities.
• Deliver the drug in the small particle size that enhances the
entire surface area of the drugs allocating quicker dissolution
in the blood.
• The concentration seems to persist at the sites for the longer
periods
• Shows EPR (enhanced permeation and retention) effect,
i.e., enhanced permeation through the barriers because of
the small size and retention due to poor lymphatic drainage
such in tumor.
• Exhibits passive targeting to the disease site of action without
the addition of any particular ligand moiety.
• Decrease in the side effects.
• Decrease in the dose of the drug formulation.
Types of Nanoparticals:
• Polymeric nanoparticles• Solid lipid nanoparticles
• Magnetic nanoparticles
• Metal and inorganic nanoparticles
• Quantum dots
• Polymeric micelles
• Phospholipids micelles
• Colloidal nano-liposomes
• Dendrimers
Nanotechnology can be used for more efficient drug delivery
system to tumors. One of the significant approach is passive
liposomal drug delivery to cancer cells. Liposome molecules are
easily diffused into the cells; since their structure and cell membrane
structure can interact very well while drug uptake process. The EPR
(Enhanced Permeation and Retention) effect is the concept that
liposomes remain the bloodstream for a long time and are collected
passively from tumor cells. Via the EPR effect, concomitant in toxicity
problems of therapy are relatively solved as lower and repeated dose
of liposomal drugs. Sengupta and Sasisekhanan stated that using EPR
effect allows up to 10 times the amount of drug to be delivered to the
tumor than the free drug method. Passive liposomal drug delivery is
also observed in Reticular Endothelial System (RES) uptake [5].
In this method PEG (Polyethylene Glycol) coated liposomes
that enable the drug system to interact with hydrophilic molecules
in cell membrane with high affinity. Moreover, these liposomal
drugs with RES relatively break away immune recognition; so drugs are able to remain in bloodstream [6]. The development of
cancer nanotherapeutics has attracted great interest in the recent
decade. Cancer nanotherapeutics has overcome several limitations
of conventional therapies, such as nonspecific biodistribution, poor
water solubility, and limited bioavailability. Nanoparticles with
tuned size and surface characteristics are the key components of
nanotherapeutics, and are designed to passively or actively deliver
anti-cancer drugs to tumor cells.
As per the national cancer institute, biological processes,
including ones necessary for life and those that lead to cancer, occur
at the nanoscale. Thus, in fact, we are composed of a multitude of
biological nano-machines (Figure 1).
Passive Tumor Accumulation: An effective cancer drug
delivery should achieve high accumulation in tumor and spare
the surrounding healthy tissues. The passive localization of many
drugs and drug carriers due to their extravasations through leaky
vasculature (named the Enhanced Permeability and Retention [EPR]
effect) works very well for tumors. As tumor mass grows rapidly, a
network of blood vessels needs to expand quickly to accommodate
tumor cells’ need for oxygen and nutrient. This abnormal and poorly
regulated vessel generation (i.e. angiogenesis) results in vessel walls
with large pores (40 nm to 1 um); these leaky vessels allow relatively
large nanoparticles to extravasate into tumor masses. As fast growing
tumor mass lacks a functioning lymphatic system, clearance of these
nanoparticles is limited and further enhances the accumulation.
Through the EPR effect, nanoparticles larger than 8 nm (between
8-100 nm) can passively target tumors by freely passing through
large pores and achieve higher intratumoral accumulation. Passive
accumulation through EPR effect is the most acceptable drug delivery
system for solid tumor treatment. However, size or molecular weight
of the nanoparticles is not the sole determinant of the EPR effect,
other factors such as surface charge, biocompatibility and in vivo
surveillance system for macromolecules should not be ignored in
designing the nanomedicine for efficient passive tumor accumulation.
Active Tumor Targeting: EPR effect (Enhanced Permeability
and Retention [EPR] effect), which serves as nanoparticle ‘passive
tumor targeting’ scheme is responsible for accumulation of particles
in the tumor region. However, EPR does not promote uptake of
nanoparticles into cells; yet nanoparticle/drug cell internalization
is required for some of the treatment modalities relying on drug
activation within the cell nucleus or cytosol. Similarly, delivery of nucleic acids (DNA, siRNA, miRNA) in genetic therapies requires
escape of these molecules from endosome so they can reach desired
subcellular compartments. In addition, EPR is heterogenous and its
strength varies among different tumors and/or patients. For these
reasons, active targeting is considered an essential feature for next
generation nanoparticle therapeutics. It will enable certain modalities
of therapies not achievable with EPR and improve effectiveness
of treatments which can be accomplished using EPR, but with less
than satisfactory effect. Active targeting of nanoparticles to tumor
cells, microenvironment or vasculature, as well as directed delivery
to intracellular compartments, can be attained through nanoparticle
surface modification with small molecules, antibodies, peptides.
Passive targeting (EPR effect) is the process of nanoparticles
extravasating from the circulation through the leaky vasculature to
the tumor region. The drug molecules carried by nanoparticle are
released in the extracellular matrix and diffuse throughout the tumor
tissue. The particles carry surface ligands to facilitate active targeting
of particles to receptors present on target cell or tissue. Active
targeting is expected to enhance nanoparticle/drug accumulation
in tumor and also promote their prospective cell uptake through
receptor mediated endocytosis. The particles, which are engineered
for vascular targeting, incorporate ligands that bind to endothelial
cell-surface receptors. The vascular targeting is expected to provide
synergistic strategy utilizing both targeting of vascular tissue and
cells within the diseased tissue. Most of the nanotechnology-based
strategies which are approved for clinical use or are in advanced
clinical trials rely on EPR effect. It is expected that next generation
nanotherapies will use targeting to enable and enhance intracellular
uptake, intracellular trafficking, and penetration of physiological
barriers which block drug access to some tumors (Figure 2).
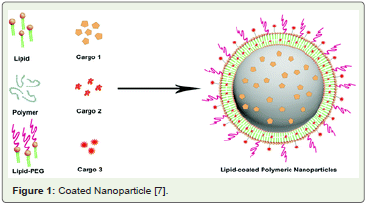
Figure 1: Coated Nanoparticle [7].
Transport across Tissue Barriers: Nanoparticle or nano-drug
delivery is hampered by tissue barriers before the drug can reach the
tumor site. Tissue barriers for efficient transporting of nano-drugs to
tumor sites include tumor stroma (e.g. biological barriers) and tumor
endothelium barriers (e.g. functional barriers). Biological barriers
are physical constructs or cell formation that restrict the movement
of nanoparticles. Functional barriers can affect the transport of
intact nanoparticles or nanomedicine into the tumor mass: elevated interstitial fluid pressure and acidic environment for examples. It is
important to design nanoparticles and strategies to overcome these
barriers to improve cancer treatment efficacy.
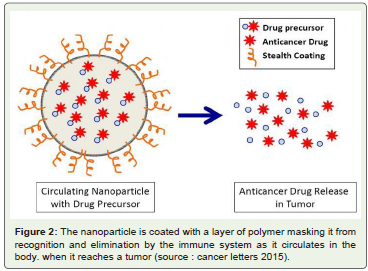
Figure 2: The nanoparticle is coated with a layer of polymer masking it from
recognition and elimination by the immune system as it circulates in the
body. when it reaches a tumor (source : cancer letters 2015).
Another formidable tissue barrier for drugs and nanoparticle
delivery is the blood-brain barrier (BBB). BBB is a physical barrier
in the central nervous system to prevent harmful substances from
entering the brain. It consists of endothelial cells which are sealed in
continuous tight junction around the capillaries. Outside the layer of
epithelial cell is covered by astrocytes that further contribute to the
selectivity of substance passage. As BBB keeps harmful substances
from the brain, it also restricts the delivery of therapeutics for brain
diseases, such as brain tumors and other neurological diseases. (NCI
.cncer and nanotech 2017). There have been tremendous efforts in
overcoming the BBB for drug delivery in general. The multi-valent
feature of nanoparticles makes nano-carriers appealing in designing
BBB-crossing delivering strategies. One promising nanoparticle
design has transfer in receptor-targeting moiety to facilitate
transportation of these nanoparticles across the BBB.
Nanoformulation in recent clinical trials: Unique properties of
nanomaterials makes available to use them as effective antineoplastic
agents or as a compound of combined therapy, in order to improve
therapeutic effectiveness of existing anti-cancer drugs. However,
despite considerable amounts of described nanotechnology-based
formulations, only a limited number of them were introduced into
clinical trials. Recently, the interest of the researchers has focused
on the employment of already used, FDA-approved nanodrugs
(Abraxane®,Genexol-PM®) as the adjuvants in combinatory therapy of
malignancies. To date, Abraxane®, e.g. paclitaxel albumin-stabilized
nanoparticle formulation (nab-paclitaxel) was approved for treatment
of metastatic breast cancer. Genexol-PM is a biodegradable cremophor
EL-free polymeric micelle formulation of paclitaxel, consisting of
poly (ethylene glycol)-poly (D, L-lactide) copolymer, with anticancer
activity [8]. The copolymer residue increases the water-solubility of
paclitaxel and allows delivery of higher doses than those achieved
with free paclitaxel. Pre-clinical in vivo studies with Genexol-PM
demonstrated a 3-fold increase in the Maximum Tolerated Dose
(MTD) and a significantly increased antitumor efficacy compared to
the free paclitaxel. In phase II clinical studies conducted in patients
suffering from metastatic breast cancer, Genexol-PM was found to be
effective and safe with high response rates [9,10].
The highly active combination of cyclophosphamide,
bortezomib, pegylated liposomal doxorubicin, and dexamethasone
was also well tolerated by patients with multiple myeloma [11,12].
Notably, the enrollment of pegylated liposomal doxorubicin
with cyclophosphamide followed by paclitaxel was safe even for
patients prone to cardiotoxicity. Moreover, treatments of peritoneal
malignancies with nanoparticulate paclitaxel have not induced toxic
effects with low peritoneal clearance of drug preserved at the same
time.
Conclusion and Prospects
It is undeniable that nanotechnology provides a variety of novel
therapeutic options applicable in the treatment of solid tumor and
hematological malignancies. However, this enthusiasm must be suppressed due to numerous reports on the considerable limitations
facing nanotechnology-based anti-cancer therapies. First of all,
physicochemical properties of tested nanomaterials (i.e. its size,
surface properties, zeta potential) influences greatly the stability in
physiological fluids, their polydispersity, binding to blood proteins
and associated efficiency of designed nanoformulation. However,
the tumor accumulation and pharmacokinetics properties are not
so easily to predict, even when the same polymers and elements of
nanosystem are used.
Overall, there is urgent need to recognize the exact properties of
nanoparticles, which permit for maximum uptake and accumulation
of drug in the target tissues. Importantly, the unique properties of
nanomaterials do not only condition their employment in therapy
of cancers, but are also responsible for a variety of toxic effects.
Despite the fact, that immobilization of anti-cancer agents on the
surface of nanomaterials should improve their biocompatibility, it is
confirmed that some nanoparticles can cause toxic effects in healthy
cells. However, the unprecedented behavior of materials used for
nanoparticle formulations, such as off-target effects or nonspecific
toxicity, maintaining consistency in particle synthesis, and controlling
penetration of biological barriers, are major hurdles to FDA approval.
Therefore, many of the nanoparticle systems that appear promising in vitro may not be successful in vivo.
Proper standards should be established for the examination
of safety and efficacy issues before expanding the newly developed
nanoparticle carriers into preclinical and clinical testing.
Implementing proper regulatory measures, a deep understanding of
tumor biology, and thoughtful use of technology advancements will
speed the possible use of these nanoparticle systems in mainstream
cancer treatment.