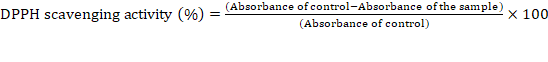Research Article
Punica Granatum L (pomegranate) Leaves Extract Exhibits Antioxidant, Anticoagulant and Antiplatelet Properties
Ramesha KL1, Sujatha M2, Shivakumar V3, Devaraja S3, and Hemalatha MS1*
1Department of Food Science and Nutrition, Karnataka State Open University, Mysuru- 570006, India
2Department of Biochemistry, Kuvempu University, Shimoga- 577451, India
3Department of Biochemistry & Department of Food Science and nutrition, Tumkur University, Tumakuru 572103, India
*Corresponding author:Hemalatha M S, Department of Food Science and Nutrition, Karnataka State Open University, Mysuru, India. Email Id: drmshemap@gmail.com
Article Information:Submission: 05/07/2024; Accepted: 31/07/2024; Published: 03/08/2024
Copyright: ©2024 Ramesha KL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Punica granatum L (pomegranate) belongs to Lythraceae family commonly known as pomegranate. The leaves of pomegranate are being extensively utilized in the folk medicine since long time. Various researchers reported the anti-inflammatory, anti-cholinesterase, anti-diabetic, anti-cancerous, cytotoxic,
anti-bacterial and antibiotic properties. While, none of the studies claims the therapeutic utility of pomegranate in oxidative stress induced pathogenesis in specific thrombosis. Thus, the current study evaluates the antioxidant and antithrombotic properties of Punica granatum Leaves Aqueous Extract (PGLAE). Qualitative analysis of PGLAE exposed the presence of carbohydrates, tannins, alkaloids, flavonoids, steroids and polyphenols. PGLAE displayed about 95% of DPPH scavenging activity with an IC50 value of 4.5μg/ml. Interestingly PGLAE significantly (***p<0.0001) normalized the stress markers such as Lipid Peroxidation (LPO), Protein Carbonyl Content (PCC), Total Thiol (TT), endogenous antioxidant enzymes such as Superoxide dismutase (SOD) and catalase (CAT) in sodium nitrite induced oxidative stress in RBC. In addition, PGLAE caused strong anticoagulation in platelet rich plasma by extending the clotting time from control 181sec to 1120sec. The anticoagulant effect of PGLAE was also strengthened by APTT and PT tests. PGLAE was positive for only APTT test, revealed the identified anticoagulant effect of PGLAE could be due its interference in intrinsic pathway of blood coagulation cascade. PGLAE also exhibited antiplatelet activity by inhibiting ADP induced platelet aggregation with an inhibition percentage of 74%. PGLAE was nontoxic in nature as it was devoid of hemolysis of RBCs.
Keywords:Oxidative stress; Antioxidant; Anticoagulant; Antiplatelet properties
Introduction
Pomegranate (Punica granatum), belongs to the family Lythraceae
commonly known as pomegranate and it is native to Asia. Perhaps, it is
broadly distributed throughout the India and major commercial crop
of southern India. It is to note that fruits, fruit peel, flowers, seeds and
leaves of pomegranate stores batteries of phytochemicals attributed
to potential health benefits [1]. The fruit of pomegranate have been
extensively consumed due to its immense therapeutic property.
While, the pomegranate leaves have been used in the folk medicine
since olden days to treat sore throat infections, thrush and urinary
tract infections, fever, flu, and pneumonia [2,3]. Although, it is richest
source of plethora of phytochemicals, researchers documented that
pomegranate found contains high number of phenolic compounds
responsible for antioxidant, antimicrobial, anti-inflammatory, antidiabetic,
and anticancer properties [4-6]. In addition, leaves also
the key reservoir of phenolic compounds, fatty acids, and essential
oils, responsible for radio-protective, peroxidation, inhibition of lipid
oxidation and antioxidant property. Thus, leaves are also extensively
used in the food industry for the production of preservatives [7,8].
Therefore, pomegranate leaves have been grasping the attention of
scientists to validate their hidden therapeutic potential in managing
various infectious and noninfectious diseases.
Reactive oxygen species (ROS) and Reactive nitrogen species
(RNS) formed during oxidative stress are typically considered as
harmful, toxic, mutagenic and carcinogenic [9]. Thus, oxidative
stress is the key cause for life style diseases such as, diabetes mellitus,
arthritis, cardiovascular complications (stroke, heart attack and
thrombosis), cancer and thrombosis [10]. High frequency of deaths
has been documented due to cardiovascular complications such as
stroke and heart attack. Recent reports suggest that the thrombotic
disorders are account for 1 in 3 deaths worldwide [11]. ROS and RNS
at higher level not only alter the functions of platelets and coagulation
factors but also damage the RBC and WBCs by generating RBC and
WBC mediated ROS [12]. Generally, ROS induced eryptosis leads
to membrane asymmetry, externalization of phosphatidyl serine
and heme leakage turned out to into prothrombotic phase [13]. High
rate of heme released from damaged red blood cells consider to be
not only cytotoxic but also activates primary hemostasis (platelets)
through endothelial damage by recruiting nitric oxide [14].
In a way, combitorial effect of ROS, RNS and cellular ROS are
the major elicitors of thrombosis and other life style diseases [15].
Hence, it is an urgent need for the identification of new therapeutic
agents for the treatment of oxidative stress and thrombosis. Perhaps,
antithrombotic drugs that are currently available in the market
tend to cause severe life-threatening side effects [16]. The plant based
medicines are also an ancient method to treat many diseases.
Hence, current study aims to characterize key phytochemicals of
Punica granatum Leaves Aqueous Extract and its antioxidant and
antithrombotic properties.
Materials and Methods
Reagents:
1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4-dinitrophenylhydrazine
(DNPH), Trichloro acetic acid (TCA), 95% ethanol,
Sodium nitrite (NaNO2), acetic acid, Thiobarbituric acid, Tetra
Methyl Ethylene Diamine (TEMED), Ethylene Diamine Tetra Acetic
Acid (EDTA), Hydrogen peroxide were purchased from Sigma
Chemical Company (St. Louis, USA). Adenosine diphosphate (ADP)
and Epinephrine were purchased from Sigma Chemical Company (St.
Louis, USA). The human blood samples obtained from the healthy
donors for the platelet‑rich plasma (PRP).Preparation of Punica Granatum Leaves Aqueous Extract (PGLAE):
Punica granatum leaves were collected from Komalapura village,
Periyapatana taluk, Mysore district. About 50g of leaves were washed
thoroughly, using grinder (SHARP, Japan), leaves were powdered.Then using 500ml of double distilled water the powder was mixed
properly, centrifuged at 1500g for 20min at 15°C and collected the
supernatant. Then the obtained supernatant was lyophilized. The
dried Punica granatum Leaves Aqueous Extract (PGLAE) was kept
at 4°C. Required amount of powder was weighed and dissolved in
double distilled water for further studies.
Qualitative analysis:
PGLAE was subjected for qualitative analysis using the method of
Harbone et al., [17] as follows.Test for carbohydrates, proteins, lipids: About 1ml of PGLAE was treated with few drops of alcoholic α-naphthol solution in a test
tube. The appearance of violet ring at the intersection indicates the
presence of carbohydrates. The PGLAE (1ml) was treated with 10%
NaOH solution followed by the addition of few drops of copper sulfate
(CuSO4) solution. The development of violet pink color indicates
presence of protein. About 1ml of PGLAE was treated with alcoholic
KOH (0.5N) followed by the addition of 1 drop of phenolphthalein
indicator. The solution was heated in water bath for 1h, the noticed
white color foam indicates the presence of lipids.
Test for alkaloids, tannins, flavonoids and phenol: Briefly 1ml of PGLAE was added with a few drops of Hager’s reagent (saturated
solution of picric acid). The existence of alkaloids indicates the
formation of yellow precipitation. About 1ml of PGLAE was received
with 1% gelatin solution having sodium chloride. The development
of white precipitate suggests the existence of tannins. About 1ml of
PGLAE was mixed with a few drops of acidic anhydride thereafter
boiled and cooled. A few drops of concentrated sulfuric acid were
added by sides of the test tubes, the formation of brown ring at the
intersection of the two layers represent steroids in the test tube
mixture. Approximately, 1ml of PGLAE was added with sulfuric acid
and formation of orange color indicates the presence of flavonoids.
About 1ml of PGLAE was added with 5% ferric chloride solution and
the development of deep blue or black color indicate the presence of
phenol.
Glycoside’s test and Triterpenoid’s test: The PGLAE (1ml) was hydrolyzed with concentrated H2SO4 for 2h in a water bath and
filtered. The filtrate was shaken with chloroform followed by the
addition of 10% ammonia. The formation of pink color indicates the
amount of glycoside present in the sample. Briefly 1ml of PGLAE was
added and shaken with concentrated sulfuric acid, the formation of
color at the lower layer indicates the presence of triterpenoids.
Determination of the antioxidant activity by the DPPH assay:
The method of Okoh et al., [18] was followed to carry out the DPPH
radical scavenging assay. A DPPH radical solution was prepared by
using 95% ethanol. The different concentration of PGLAE (5-30μg)
was mixed with 150μL of DPPH radical solution and the final volume
was made to 600μL by using 95% of ethanol and incubated for 30min
at 37℃. The absorbance of the reaction mixture was measured by
using spectrophotometer at 517nm. Ethanol was used as a blank and
ascorbic acid was used as a positive control. The radical scavenging
activity was calculated by using the given below formula.Oxidative stress induction in HRBCs by using sodium nitrite (NaNO2) inducer:
The method of Luqman and Rizvi et al., [19] was followed to
induce the oxidative stress in HRBCs model. In briefly, 1ml of
washed RBC suspension was preincubated with different doses of
PGLAE (20-80μg) for 30min in a clean test tube. Then 20μl of NaNO2
(10mmol/l) was added to the test tubes and further rested for 90min
at 37℃. The NaNO2-untreated RBC was considered as positive
control and NaNO2-treated RBC (absence of PGLAE sample) was
taken as a reference control. Lastly, 2mg/ml of RBC lysate was taken
from each tube and measured the level of lipid peroxidation (LPO),
protein carbonyl content (PCC), Total Thiol (TT) and antioxidant
enzyme (superoxide dismutase and catalase) activity.Determination of Lipid peroxidation (LPO):
The method of Ohkawa et al., [20] was followed to estimate the
lipid peroxidation. Briefly, 100 μL of (10mM) NaNO2 was mixed
with RBCs incubated with PGLAE then about 1.5mL of acetic acid
(pH 3.5, 20% v/v), SDS (8% w/v, 0.2mL) and 1.5mL thiobarbituric
acid (0.8% w/v) added to the reaction mixture and were boiled at
45-60℃ for 45min and centrifuged at 2000rpm for 10min. The
formed adducts were extracted by using 3ml of 1-butanol. The TBRS
(Thiobarbituric acid-reactive substance) in the supernatant was
measured by spectrophotometrically (Thermo Scientific Biomate 6,
USA) at 532nm. The values were stated in terms of malondialdehyde
(MDA) corresponding to μmol MDA formed/mg of protein.Determination of Protein Carbonyl Content (PCC):
The method of Levine et al., [21] was followed to assess the protein
carbonyl content. Briefly 100μL of 10mM NaNO2 was treated with
RBC preincubated with PGLAE in a clean test tube. Subsequently,
an equal amount of 10mM 2,4-dinitrophenylhydrazine (DNPH)
was added in a 2mmol/L HCl mixed well and left for 1h at room
temperature. Only 2mmol/L HCl was used for blank. To obtain the
precipitation of the reaction mixture, TCA (20%, w/v) was added and
centrifuged at 1200rpm for 15min. the precipitate from the reaction
mixture was cleaned using acetone and centrifuged for 15min at
2500rpm to get the final pellet. At the end, 20mM of 1ml Tris buffer
[pH 7.4 having 0.14M NaCl, 2% SDS (w/v)] was used to melt the pellet
and OD of the supernatant was estimated spectrophotometrically
(Thermo Scientific Biomate 6 USA) at 360nm. The results were
expressed as μmol carbonyl groups/mg of protein.Measurement of total thiols (TT):
The method of Zinellu, et al., [22] was used to assess the total thiol
content. In briefly, 100μl of 10mM NaNO2 was treated with RBCs
which are preincubated with PGLAE in a clean test tube. About
0.375ml of 0.2M Tris-HCl buffer (pH 8.2) was added and vortexed.
The reaction mixture was incubated for 30min by adding 10mM
dithiol-bis-nitro benzoic acid (DTNB) and 1.975ml of methanol. The
reaction mixture was centrifuged at 5000rpm for 10min. The samples’
clear supernatants were collected and measured for photometric
absorbance at 412nm, with the thiol content expressed as nmol of
DTNB oxidized/mg protein.Superoxide dismutase (SOD) activity:
The method of Sundaram et al., [23] was followed to assess the
SOD enzyme activity. In briefly, about 0.05mg protein from RBC
lysate containing PGLAE (20-80μg/ml) with an agonist 10mM
NaNO2 taken in a clean dry test tube. The reaction mixture was mixed
with 1ml of 16mM phosphate buffer (pH 7.8) containing TEMED–
EDTA (8mM/0.08mM) mixture. The decrease in absorbance was
evaluated spectrophotometrically (Thermo Scientific Biomate 6 USA)
at 406nm. The obtained result was expressed in U/mg of protein.Catalase (CAT) activity:
The procedure of Beers et al., [24] was followed asses the CAT
enzyme activity. In briefly 0.05mg of protein lysate from RBC treated
with PGLAE (20-80μg/ml) was taken in a clean, dry test tubes. The
reaction mixture added with 1ml of 100mM phosphate buffer (pH
7.4) and 8.8mM H2O2. The optical density of the reaction mixture was
measured spectrophotometrically at 240nm and the CAT activity was
expressed as U/mg of protein.Plasma re-calcification time:
The assay was carried out according to the method of Quick et
al., [25]. The crude PGLAE (2-12μg) was treated with PRP in 10mM
20μL tris HCl buffer of pH 7.4 for 1min at room temperature. The
incubated mixture was treated with 20μL of 0.25M CaCl2 and the
clotting time was recorded.PT and APTT:
As described by the method of Gangaraju S et al., [26] activated
partial thromboplastin time and prothrombin time were carried out.
The PGLAE (2-10μg/ml) was pre-incubated with 100μl of human
citrated plasma for 1min. For APTT, 100μl of LIQUICELINE
(Cephaloplastin derived from rabbit brain with phospholipids and
ellagic acid preparation) was activated for 3min at 37°C. The clotting
time was measured by adding 100μl of 0.02M CaCl2. For conduct
Thromboplastin (PT), the clotting time was initiated by adding 200μl
of PT reagent. The clot formation time was recorded in seconds.Platelet aggregation:
According to the method of Born [27], the turbidimetric approach
was used inspect the impact of PGLAE on platelet function by using
chronology dual channel whole blood optical lumi aggregation
system. The varied concentration of PGLAE (20–60μg) and PRP
(0.25ml) were preincubated and agonist was started by adding 10μM
ADP and aggregation was monitored for 6min.Direct hemolytic activity:
Briefly, 1ml of erythrocyte and 9ml of PBS were mixed thoroughly.
About 1ml of hematocrit suspension was incubated with PGLAE
(50-200μg) at 37℃ for 1h. The reaction mixture was added with
9ml of ice-cold PBS to terminate the reaction. The reaction mixture
was centrifuged at 1500rpm for 10min. The release of hemoglobin
in the supernatant was measured by spectrophotometer (Thermo
Scientific Bio-mate 6, USA) at 540nm and percentage of hemolysis
was calculated and plotted.Results
PGLAE exhibited antioxidant activity due to stored plethora of secondary metabolites:
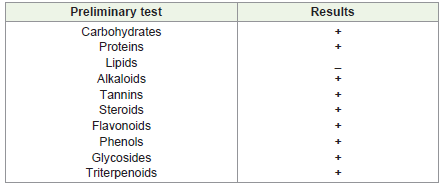
The initial screening of PGLAE was done by qualitative analysis
of phytochemicals. The study revealed the presence of alkaloids,
tannins, steroids, polyphenols, glycoside and triterpenoids (Table1).
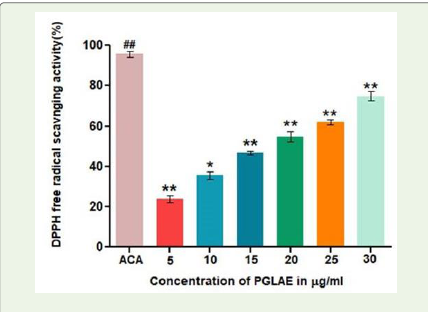
Thus, antioxidant activity of PGLAE was carried out, interestingly,
PGLAE exhibited antioxidant activity by scavenging DPPH radical in
a dose dependent manner. PGLAE showed about 70% radical with an
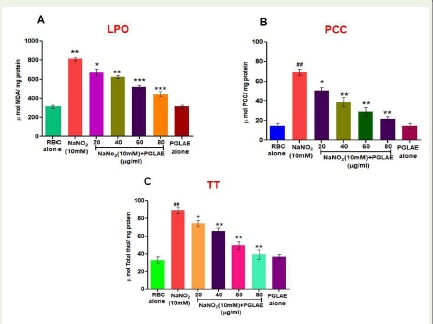
IC50 value 4.94μg/ml [Figure 1].PGLAE regulates NaNO2-induced oxidative stress marker in-vitro:
The level of lipid peroxidation was measured as liberation of
malondialdehyde (MDA) content. In case of NaNO2 treated RBCs
the level of MDA was significantly (P< 0.001) increased. Whereas
in PGLAE treated RBCs, the level of MDA was significantly (P<
0.001) normalized as compared to normal RBCs alone in a dose
dependent manner [Figure 2A]. Similarly, the protein carbonyl
content in NaNO2 treated RBCs was elevated compare to the normal.
But in case of PGLAE treated RBCs there was a significant (P<0.001)
decrease in the level of carbonyl content was noticed [Figure 2b].
Similarly, the total thiol content was also increased in NaNO2 treated
Figure 1:DPPH scavenging activityof PGLAE: Antioxidant activity of PGLAE
measured by DPPH method. ACA: Ascorbic acid.
RBCs, however, in PGLAE treated RBCs there was a significant
decrease in the level of thiol content was identified [Figure 2C].
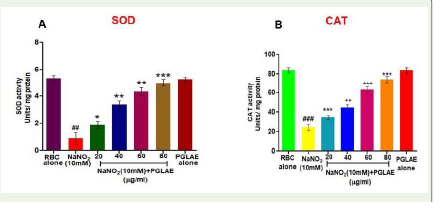
Furthermore, PGLAE was also normalized the anti-oxidant enzymes
such as superoxide dismutase (SOD) and catalase (CAT) activities in
NaNO2-treated RBCs. In the case of NaNO2-treated RBCs, there was
a significant (P< 0.01) decrease in the SOD and CAT activities [Figure 3A,3B]. Perhaps, PGLAE restored the SOD and CAT activity
in a dose-dependent manner with a statistically significant value at
the concentration of 80μg (P< 0.01& P< 0.001) respectively.
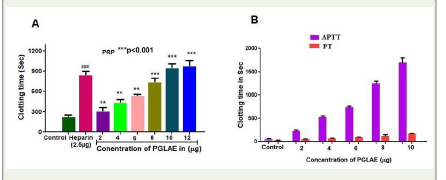
PGLAE exhibited anticoagulant property by interfering with intrinsic and extrinsic pathway of blood coagulation:
PGLAE exhibited anti-coagulation by increasing the clotting time
of PRP control 200s to 1010s [Figure 4A]. PGLAE delayed the clotting
time of both APTT and PT suggesting its anti-coagulant effect was
due to the interference in both intrinsic and extrinsic pathway of
blood coagulation cascade [Figure 4B].
Figure 2:Effect of PGLAE on oxidative stress markers: (A) LPO, (B) PCC,
(C) TT in RBCs. For determination of oxidative stress markers, NaNO2
(10mmol/L) was used as an inducer.Data are presented as mean ± SD (n =
3). [n = 3, p<0.05 (*), p<0.01 (**), p<0.001 (***/###); #: significant compared
to untreated RBCs, *significant compared to NaNO2 (10mM) treated RBCs.
Figure 3:Effect of PGLAE on antioxidant enzymes: (A) SOD (B) CAT in
RBCs. For determination of oxidative stress markers, NaNO2 (10mmol/L)
was used as an inducer.Data are presented as mean ± SD (n = 3). [n =
3, p<0.05 (*), p<0.01 (**), p<0.001 (***/###); #: significant compared to
untreated RBCs, *significant compared to NaNO2 (10mM) treated RBCs.
Figure 4: (A) Plasma re-calcification time of PGLAE: PGLAE (2-12μg)
was pre-incubated with 0.2ml of citrated human plasma PRP/PPP in the
presence of 20μl 10mM Tris–HCl buffer (pH 7.4) for 1min at 37°C. 20μl of
0.25M CaCl2 was added to the pre-incubated mixture and clotting time was
recorded. (B) APTT and PT: PGLAE (2-10μg) was pre-incubated with 0.2ml of normal
citrated human plasma for 1min at 37°C. 100μl of APTT reagent which was
activated for 3min at37°C, was added. The clotting was initiated by adding
100μl of 0.02M CaCl2 and the clotting time was measured. For PT: The
clotting time was initiated by adding 200μl of PT reagent to PGLAE (2-10μg)
the time taken for the visible clot was recorded in seconds.
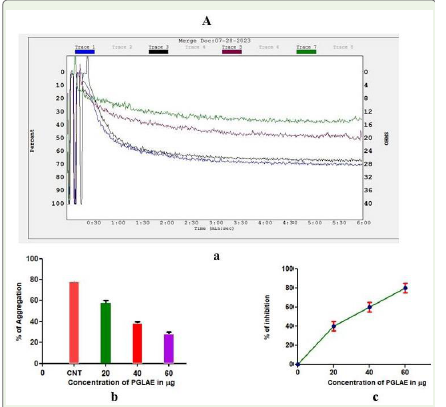
PGLAE exhibited anti-platelet activities:
PGLAE was scrutinized for platelet aggregation properties by
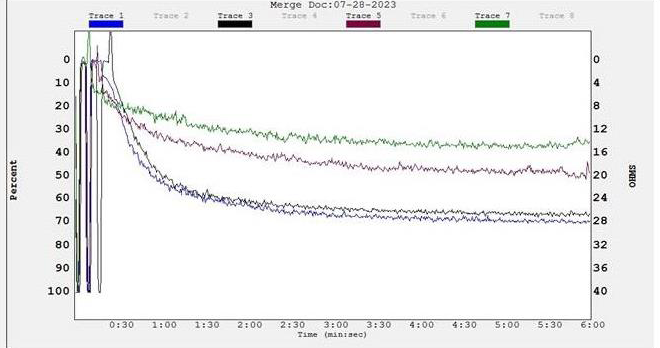
using ADP as an agonist. PGLAE inhibited ADP induced platelet
aggregation of platelet rich plasma in a concentration dependent
manner with the aggregation inhibition of 70% at the concentration
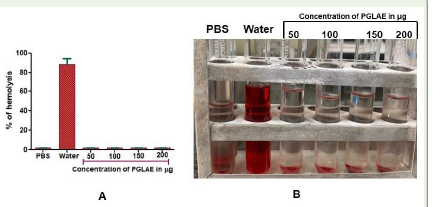
of 60μg [Figure 5].PGLAE displayed non toxicity to RBCs:
The PGLAE did not rupture the RBCs cell membrane, whereas,
water a positive control ruptured the RBCs and generated the ghost
red blood cells revealed its nontoxic nature [Figure 6].Discussion
Higher level of free radicals generated during biological oxidation
found to elicit cytotoxicity, as they damage proteins, lipids and DNA
[28]. Thus, oxidative stress is the key cause for the pathogenesis of
all types of life style diseases such as, diabetes, thrombosis, cancer,
and autoimmune diseases and neurological syndromes. The life style
diseases have been managed by non-steroidal, anti-inflammatory
and steroidal drugs. It is to note that the said drugs trigger severe
side effects such as headache, painful periods, sprains and strains,
colds, flu and arthritis with long term pain [29]. Thus, researchers
thriving to explore an alternative therapy. Perhaps, drugs discovered
from medicinal plants appear to be promising as they are devoid
of cellular toxicity but having high range of therapeutic index.
Although, medicinal plants have been exploited since ancient time
to treat several ailments, several such medicinal plants have not
been validated scientifically. Thus, in the current study antioxidant
and antiplatelet activity of Pomegranate (Punica granatum) leaves
was undertaken. Pomegranate (Punica granatum) belongs to the
Lythraceae family and it has been extensively used traditional herbal
medicine [30].
The various parts of pomegranate found to exhibit antiinflammatory,
anti-oxidant, anti-cholinesterase, and cytotoxic
properties, anti-bacterial and antibiotic properties were reported
Figure 5:Inhibition of ADP induced platelet aggregation by PGLAE:
(Aa) Traces of platelet aggregation Trace 1 (ADP 10μM); Trace 2 (ADP 10μM
+ 20μg of PGLAE); Trace 3 (ADP 10μM + 40μg of PGLAE); Trace 4 (ADP
10μM + 60μg of PGLAE). (Ab) Dose dependent platelet aggregation%. (Ac)
Dose dependent platelet aggregation inhibition%.
Figure 6:Direct Hemolytic assay of PGLAE: (A) The amount of
hemoglobin released in the supernatant was measured at 540nm and
percentage of hemolysis was calculated.
(B) The different concentration of PGLAE (50-200μg) was incubated
independently for 1h at 37°C with the 1ml of suspension made with packed
human erythrocytes and phosphate buffered saline (PBS) 1:9 v/v.
[31]. According to our qualitative tests pomegranate leaves gave
positive results for the secondary metabolites such as alkaloids,
tannins, steroids, polyphenols, glycosides, and triterpenoids. During
oxidative stress there could be a massive decrease in the production
of endogenous antioxidant machineries, which has to be managed
by providing antioxidant load externally.
Interestingly PGLAE exhibited potential antioxidant property by
quenching DPPH free radical. Plant extract such as, Hyptis fasciculata,
Orbignya speciosa [30], Paeonia suffruticosa, Cinnamomun cassia [31] found to exhibit antioxidant activity. Free radicals stored in the body
affect all the cells, however, red blood cells are more sensitive and often
undergo eryptosis by free radicals, generates RBC mediated reactive
oxygen species which are not only damage WBC and platelets but also
linked to thalassemia, sickle cell anemia, diabetes, sepsis, hepatic and
renal insufficiency, Wilson’s disease, hemolytic uremic syndrome,
hypophosphatemia, G6PD-deficiency, chronic kidney disease and
heart attack [32]. Our findings revealed that PGLAE normalized the
sodium nitrite-induced stress markers such as LPO, PCC, TT, SOD,
and CAT in RBCs. Therefore, PGLAE may be a better candidate in the
management of oxidative stress induced pathogenesis. Plants extract
such as, sorghum extract protects RBC from sodium nitrite-induced
oxidative stress [33].
ROS and ROS mediated cellular ROS alters hemostasis (platelets,
coagulation cascade and fibrinolysis) is main cause for thrombosis
what is known as formation of unusual clot in the arteries and
veins [34]. Antithrombotic drugs (anti-coagulant, antiplatelet
and fibrinolytic) have been currently using in the management
of thrombotic drugs. The recent reports suggests that the said
antithrombotic drugs triggers side effects such as passing blood urine,
passing blood in the poo, bruising, prolonged nosebleeds, vomiting
blood and severe backpain [35]. Hence their utility is also considered
to be the bottleneck. Importantly, PGLAE showed anticoagulant
property by extending the clotting time of platelet rich plasma (PRP).
In addition, PGLAE also extended the clotting time of both APTT
& PT clearly indicates that the examined anticoagulant potential
of PGLAE is due to the interference both intrinsic and extrinsic
pathway of blood coagulation cascade non-specifically. The plant
extract such as Angelica shikokiana [36], Cyclopia subternata [37],
Thymus atlanticus [38] exhibited anticoagulant activities. Platelets
are the major components of connective tissue play an important
role in maintaining fluidity of the blood. Often their damage by
ROS, bacteria, toxins and drugs activates them to initiate a clot [39].
Thus, platelet activation contributes immensely in progression of
thrombotic disorders (heart attack and stroke) [40]. Antiplatelet
interventions ought to be the treatment option for the prevention of
thrombotic disorder [41]. Surprisingly, PGLAE also inhibited agonist
ADP triggered platelet aggregation of human platelet rich plasma
confirms its antiplatelet activity. Plant extract such as, Selaginella
bryopteris [42] Lindera obtusiloba [43] and Leuzea carthamoides [44]exhibited antiplatelet activities. Moreover, PGLAE was unable to
damage RBC compare to positive control water revealed its non-toxic
nature.
Conclusion
In conclusion, we for the first time explored the potential
therapeutic role PGLAE. PGLAE through its antioxidant potential
ameliorates sodium nitrite oxidative induced RBC damage. In
addition, PGLAE showed anticoagulant and antiplatelet properties.
Hence, purification and characterization of the actives principles is of
great future interest.
Conflict of interest:
The authors declare that there are no conflicts of interest