Research Article
Elettaria Cardamomum Seed Extract Exhibits Antioxidant and Procoagulant Activities
Sujatha M Hanumegowda, Yamuna RS, Kavya P, Riya raj, Chandrakant S Karigar, Manjunatha H, Chandramma Dayananda* and Prathiba HD
Department of Biochemistry, Bangalore University, Jnanabharathi Campus, Bangalore, Karnataka, India
*Corresponding author:Chandramma Dayananda, Department of Biochemistry, Bangalore University, Jnanabharathi Campus, Bangalore, India. E-mail Id: drchandramma@bub.ernet.in
Copyright: © 2024 Sujatha M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article Information:Submission: 20/10/2024; Accepted: 18/11/2024; Published: 21/11/2024
Abstract
Elettaria cardamomum seeds stores robust phytochemicals responsible for several therapeutic efficacy, hence it is a rich source of useful components that has medicinal properties and one of the main applications is in controlling bleeding. Thus, current study investigates the antioxidant, procoagulant and clot fibrinolytic efficiency of Elettaria cardamomum Seed Aqueous Extract (ECSAE). The antioxidant potential of the extract was assessed by antioxidant
activity using 2, 2 - diphenyl, 1- picryl hydrazyl (DPPH) radical scavenging activity. The ECSAE was found to have 63.00% of DPPH scavenging activity with an IC50 value of 45.48μg/ml compare to positive control ascorbic acid. Preliminary phytochemical screening was analyzed by instrumentation method such as RP-HPLC and GC-MS. The protein blue prints of ECSAE revealed similar banding pattern from 15-200kDa on 10% SDS-PAGE suggested that ECSAE reserves only monomeric proteins. ECSAE exhibited proteolytic activity by degrading casein. The specific activity was fond to be 0.27 units/mg/min at 37°C. The proteolytic activity was strongly inhibited by both PMSF and IAA. While, EDTA and 1,10 Phenanthroline did not show inhibition, suggesting the presence serine and cysteine protease in the seeds. ECSAE showcased procoagulant effect as reduced the clotting time of PRP from control 214.2s to 94.2s which promotes blood coagulation. ECSAE found to hydrolyze fibrin clot with specific activity of 1.1 to 4.5units in a dose dependent manner. Furthermore, ECSAE did not hydrolysis the RBC suggested its non-toxic properties.
Keywords:Elettaria Cardamomum; Protease; Antioxidant; Procoagulant
Introduction
Haemostasis, coagulation, and fibrinolysis systems are those that
are part of the physiological system that maintain the human body
functioning normally. By restricting one another, the haemostatic
system preserves homeostasis. When deviations in a single system’s
operation may result in bleeding or thrombosis, which may then lead
to a number of illnesses, including vascular disease and haemophilia
[1]. A class of inherited hemorrhagic diseases with coagulation issues
is known as haemophilia. The dysfunction of active thrombozyme,
the extension of the coagulation time, the enduring propensity
to bleed following small trauma, and “spontaneous” bleeding in
severe individuals who have not experienced evident trauma are the
common hallmarks of haemophilia [2]. Haemophilia is mostly treated
with local haemostatic therapy and other therapies; nevertheless,
there are notable side effects, including an increased risk of hepatitis,
haemolysis, and acquired immunodeficiency sickness (AIDS), and a
poor cure rate. Vasoconstriction and platelet response, coagulation
and anticoagulation systems, and the fibrinolytic system are the
three interrelated components of the complicated physiological,
biochemical, and pathological process that makes up normal
physiological procoagulant and anticoagulant mechanisms [3]. The
body’s ability to coagulate normally is mostly based on the whole
vascular wall’s structure and function, the quantity and quality of
functional platelets, and the regular activity of plasma coagulation
factors. Anticoagulation and fibrinolytic systems also have an impact
on the coagulation and anticoagulation systems [4]. Numerous
studies have been conducted on medications that activate clotting
factors and promote platelet coagulation blood to successfully halt
bleeding but their use is restricted by the side effects[5]. Nowadays,
procoagulant-active natural compounds are a major topic in research.
Medicinal plants are a massive and vital source of knowledge for
indigenous medical systems. All most all part of plants have been
using for therapeutic application but seeds from the plants have been
least explore. One of such medicinal plant is perennial (Elettaria
cardamomum). Seeds of Elettaria cardamomumare cardamom [5].
It is belonging to the ginger family (Zingibaraceae). Cardamom is
a valuable spice originates from the coastal area of India. Occurring
in Guatemala, Tanzania, Sri Lanka, El Salvador, Vietnam, Laos
and Cambodia [6]. India is the chief exporter of dried cardamom.
α-terpineol, myrcene, heptane, subinene, limonene, cineol,
menthone, α-pinene, β-pinene, linalol, nerolidol, β-sitostenone,
phytol, eugenyl acetate, bisabolene, borneol, citronellol, geraniol,
geranyl acetate, stigmasterol and terpinene were found in cardamom
[7]. In this connection, the seeds such as Azadirachta indica, Allum
cepa, Elettaria cardamomum, Myrista fragrans, Brassica rapasubsp
rapaseed extracts were screened for various biological activity.
However, Elettaria cardamomum seed extract exhibited procoagulant
activity. Thus, Elettaria cardamomum seeds have been selected for
further examinations.
Materials and methods
Phenyl Methyl Sulphonyl Fluoride (PMSF), Ethylene Diamine
Tetra-acetic Acid (ED- TA), Iodo-Acetic Acid (IAA) and
1,10-Phenanthroline were purchased from Sigma chemicals company
(St. Louis, US- A). Molecular weight markers were from Bangalore
Genie Private Limited, India. Fresh human blood was collected from
healthy donors for Platelet Rich Plasma (PRP) and Platelet Poor
Plasma (PPP).
Preparation of Elettaria cardamomum Seed Aqueous Extract (ECSAE):
Elettaria cardamomum seed were collected from were purchased
from local provisions store of Bangalore. Seeds were separated from
coat and 20% homogenized in distilled water and centrifuged at
5000rpm for 20min at 15°C. Then, the supernatant was subjected
to ammonium sulfate precipitation (30 %) and spun at 1500g for
20min. Later, the pellet was mixed with water and kept for dialysis
overnight. The protein sample was stored at -20°C until further use.
Protein concentration was determined as described by Lowry’s et al.
[8]. Using Bovine Serum Albumin (BSA) as standards.Reverse Phase High Performance Liquid Chromatography analysis:
ECSAE was subjected to RP-HPLC using C18 column
(150mm×4.60mm, particle size 5μm) with PDA detector in shimadzu
LC-20AD prominence. The column was pre-equilibrated with 0.1%
Trifluoroacetic acid (TFA) in water and it was eluted at the flow rate
of 1ml/min in linear gradient mode.GC-MS:
GC-MS analysis of samples was analysed on quadrupole
mass spectrometers in the electron capture negative-ion chemical
ionization (ECNICI) mode with capillary column (30X0.25mm
IDX1EM df, composed of 100% Dimethyl poly siloxane). Helium
(99.9%) gas was used as carrier gas at the flow rate of 1ml/min and the
injection volume of 0.5 El (split ratio of 10:1). Temperature program
was set as follows, injector temperature 250°C; ion-illuminator
temperature 280°C, oven temperature 110°C (isothermal for 3min)
with an increase in temperature of 20°C/min to 220°C, thereafter 5°C/
min to 300°C. Mass spectrum was taken at 80ev; a scan interval of
0.5s [12].Determination of the antioxidant activity by the 1,1- diphenyl-2-picrylhydrazyl assay:
The DPPH radical scavenging activity was measured according to
the method of Okoh et al. [9]. An aliquot of 50–200mg of ECSAE was
made up to 600ml of (0.3mM) DPPH, which was dissolved in 95%
ethanol. The mixture was shaken and incubated for 30min in a dark at
room temperature. Then, the absorbance of the mixture was recorded
at 517nm. Ethanol was used as blank and ascorbic acid (1mg/ml) was
used as a positive control. The scavenging activity was calculated by
the following formula: DPPH scavenging activity (%) = Absorbance
of blank-Absorbance of sample X 100 Absorbance of blank. The
antioxidant activity of ECSAE was expressed as IC50. The IC50 value
was defined as the concentration (mg/ml) of the extract required for
inhibiting the formation of DPPH radical by 50%.Ammonium sulphate precipitation of ECSAE and Protein estimation:
The prepared extract was further used for the precipitation of
proteins and proteins were precipitated using 30% of ammonium
sulphate. Precipitated proteins were again centrifuged at 5000rpm for
2min, pellet was subjected to dialyzed overnight. The protein sample
obtained was stored at -20°C in freeze condition until further use. This
extracted protein sample was used throughout the study and referred
as ECSAE. Protein concentration was determined as described by
Lowry’s et al. [9]. Using Bovine Serum Albumin (BSA) as standards.Sodium Dodecyl Sulfate-Poly Acrylamide Gel electrophoresis (SDS-PAGE):
10% SDS-PAGE was assayed based on the method described by
Laemmli [10]. ECSAE crude (100μg) was treated in both reduced
and non-reduced reagents and electrophoresis was performed by
using Tris (25mM), glycine (192mM) and SDS (0.1%) for 2h at room
temperature. Upon that gels were stained with 0.1% Coomassie
brilliant blue R-250 thereafter de-stained with 40% ethanol in 10%
acetic acid and water (40:10:50 v/v) to visualize the protein bands.
Molecular weight standards were used from 125kDa to 24kDa.Proteolytic activity:
Analysis of proteolytic activity was assayed based on the method
described by Satake et al., [11]. ECSAE crude 80μg was incubated
with fat-free casein (0.4ml, 2% in 0.2M Tris–HCl buffer, pH 7.6) at
37°C for 2h and 3min. Add 1.5ml of 0.44M Tri-chloro Acetic acid
(TCA) in order to precipitate undigested casein and allowed to stand
for 30min. Thereafter centrifuged at 2000g for 10min. Upon that
add 2.5ml of 0.4M sodium carbonate and 0.5ml of Folin–Ciocalteu’s
reagent (1:2) to 1ml of the supernatant and the color developed were
measured at 660nm. One unit of the enzyme activity was defined as
the amount of the enzyme required to cause an increase in optical
density (OD) of 0.01 at 660nm/min at 37°C. The specific activity was
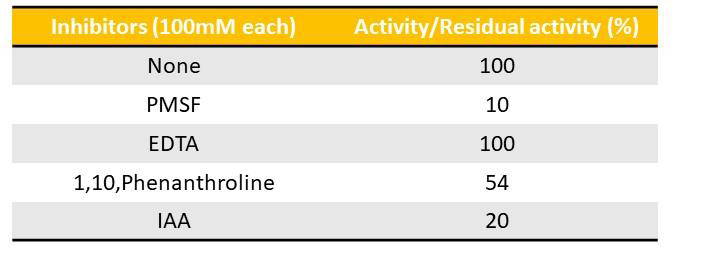
expressed as units/min/mg of protein. The inhibition studies was
performed independently by pre-incubating the crude ECSAE (80μg)
for 30min with 100mM each of EDTA, 1,10-phenanthroline, PMSF
and IAA.Preparation of Platelet Rich Plasma (PRP) and Platelet Poor Plasma (PPP):
PRP and PPP were prepared based on the method described by
Ardlie and Han [12]. The platelet concentration of PRP was adjusted
to 3.1×108 platelets/ml with PPP. The PRP maintained at 37°C was
used within 2h. PRP was prepared using plastic wares or siliconized
glass wares.Plasma re-calcification time:
The plasma re-calcification time was assayed based on the
method described by Quick et al., [13]. ECSAE crude (5-50μg) was
pre-incubated with 0.2ml of citrated human plasma in the presence of
10mM Tris HCl (20μl) buffer pH 7.4 for 1 min at 37°C.Fibrin clot-hydrolyzing activity by colorimeter:
Fibrin clot-hydrolyzing activity was assayed based on the method
described by Rajesh et al., [14]. Concisely, 100μl of citrated human
plasma was mixed with 20μl of 0.2M CaCl2 and incubated for 2h at
37°C. The clot obtained was washed thoroughly for 5–6 times with
PBS and suspended in 400μl of 0.2 M Tris–HCl buffer (pH 8.5). The
reaction was initiated by adding varied amounts of ECSAE (20–
100μg) in 100μl of saline and incubated for 2h and 30min at 37°C.
The undigested clot was precipitated by adding 750μl of 0.44M TCA
and allowed to stand for 30min.Results and Discussion
The present study was designed to investigate the antioxidant,
procoagulant and fibrinolytic activities of ECSAE. This study
reported to innumerable medicinal application of ECSAE. Recently,
considerable attention has been paid on the identification of natural
plant-derived bioactive substances (e.g., antioxidants) that may
be used in clinical routine. Many natural compounds present in
the human diet can lower the risk of developing diseases such as
cancer, cardiovascular and neurodegenerative disorders [16]. Living
organisms are equipped with enzymatic and chemical antioxidant
mechanisms for the control of oxidants. Certain amount of oxidative
damage takes place even under the normal conditions; however,
increased oxidant and decreased antioxidant levels defeat the ability
of the antioxidative mechanisms to prevent oxidative damage [17].
DPPH is stable free radical at room temperature and accepts an
electron / hydrogen radical to become a stable diamagnetic molecule.
The reduction capability of DPPH radical is determined by the
decrease in its absorbance at 5l7nm, induced by antioxidants. The
decrease in absorbance of DPPH radical is caused by antioxidants,
because of the reaction between antioxidant molecules and radicals,
progresses, which results in the scavenging of the radical by hydrogen
donation. It is visually noticeable as a change in color from purple to
yellow [18]. Hence, DPPH is usually used as a substrate to evaluate
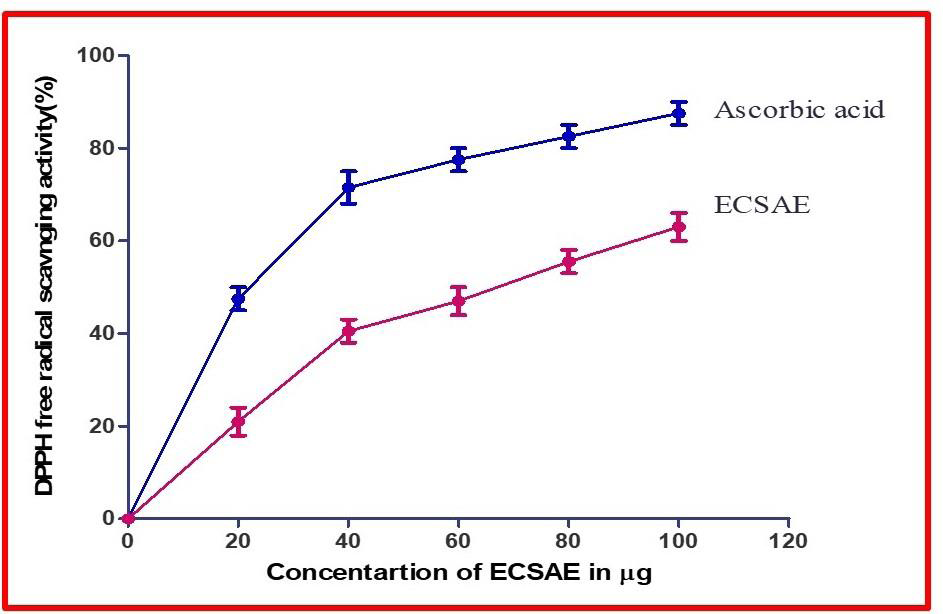
the antioxidative activity. ECSAE exhibited the 76% of DPPH radical
scavenging activities at the concentration of 100μg with an IC50 value
of 45.38μg/ml compare to positive control ascorbic acid. Ascorbic
acid showed 81.34% of DPPH scavenging activity at the concentration
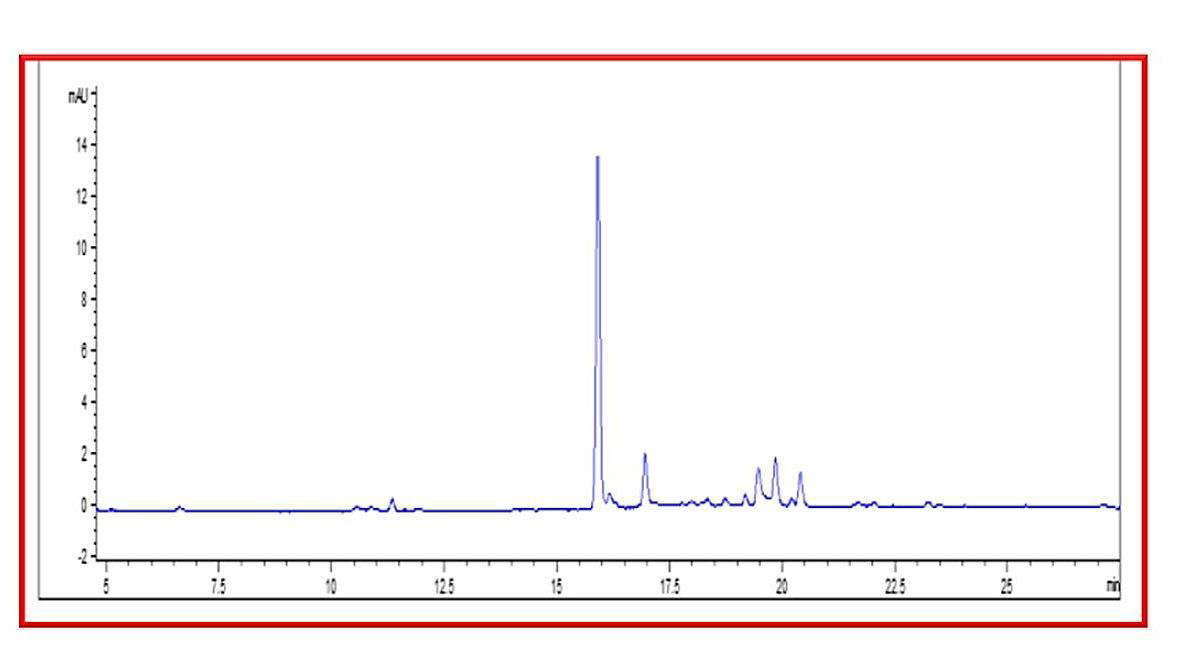
of 100μg [Figure 1]. In RP-HPLC, assessment of compound was
based on the retention time of obtained chromatogram. Five peaks
were resolved at the retention time of 16, 17, 19.5, 20 and 20.5min
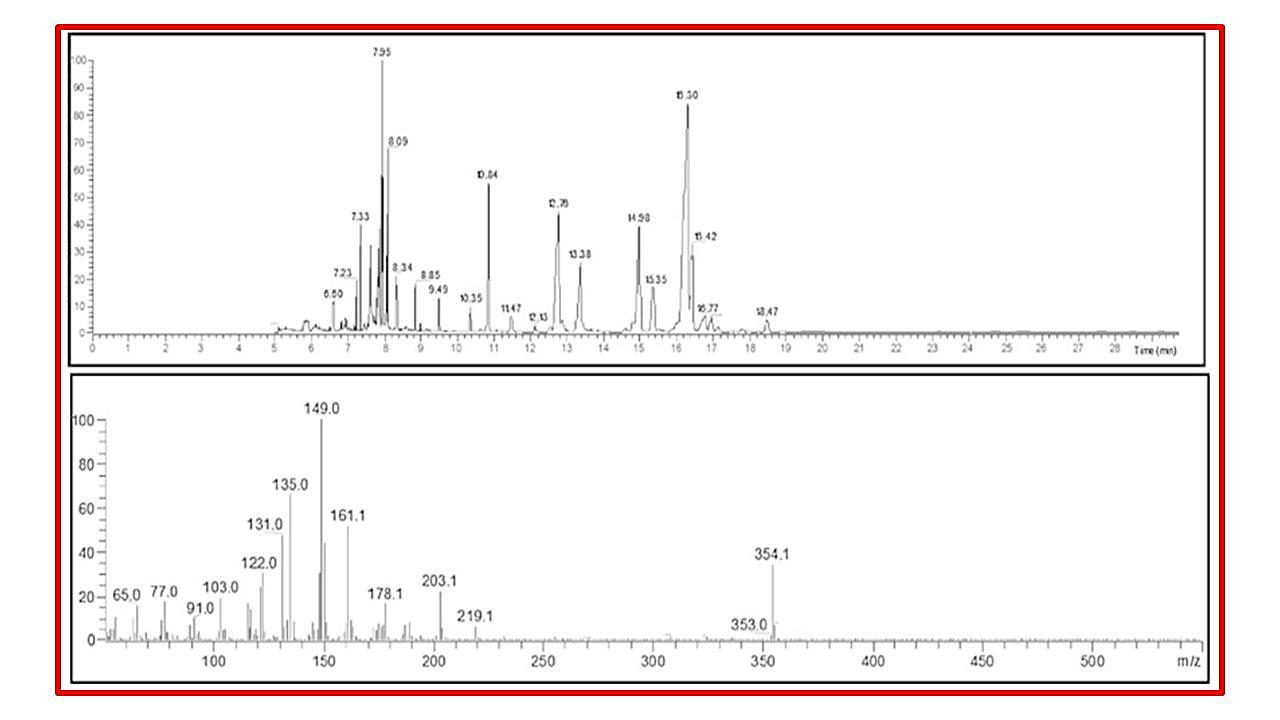
respectively [Figure 2]. GC-MS chromatogram indicated the presence
of large array of phytochemicals showed in [Figure 3]. Interestingly,
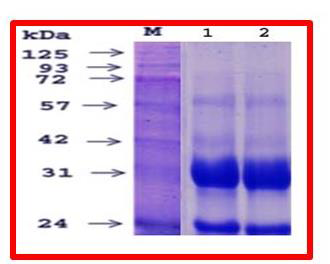
ECSAE showed the similar protein banding pattern arranged
between 24 and 125kDa on 10% SDS-PAGE under reduced and nonreduced
conditions [Figure 4]. ECSAE exhibited proteolytic activity
by degrading casein as a substrate with the specific activity of 0.270
units/mg/min at 37°C. Proteolytic activity of ECSAE was completely
neutralized by both PMSF and IAA. While, 1,10, Phenanthroline
Figure 1:DPPH Free radical scavenging activity. Antioxidant activity of
ECSAE, measured by DPPH method. Each value is presented as mean ±
standard error.
Figure 2:HPLC Chromatogram. ECSAE (20μL) was injected to C18 Column
(5mm, 0.21X25cm) which was pre-equilibrated with 0.1% Trifluoro Acetic
Acid (TFA) in water and sample was eluted in gradient mode by increasing
the concentration (0- 100%) of 0.1% TFA in acetonitrile for 20min at the flow
rate of 1ml/min and monitored at 280nm.
Figure 4:SDS-PAGE 10%: ECSAE as shown in SDS-PAGE (10%): ECSAE
(100μg) under non-reduced (a1) and reduced conditions (a2).
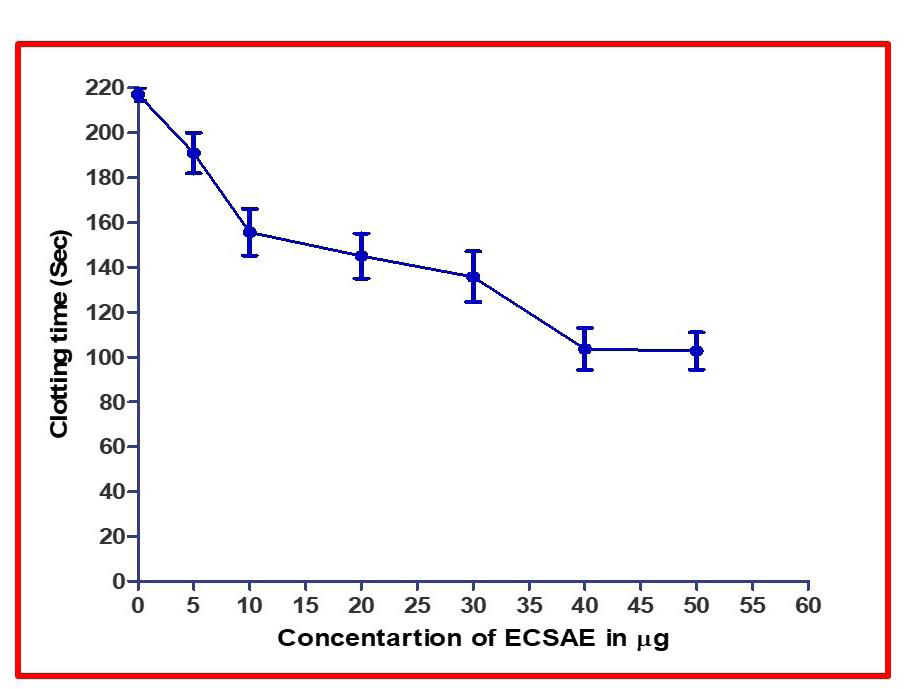
Figure 5:Plasma re-calcification time. ECSAE (5–50μg) was pre-incubated
with 0.2ml of citrated human plasma PRP/PPP in the presence of 20 μl
10mM Tris–HCl buffer (pH 7.4) for 1 min at 37°C. 20μl of 0.25M CaCl2 was
added to the preincubated mixture and clotting time was recorded.
and EDTA, have not inhibited the proteolytic activity of the extract.
This suggested that ECSAE comes under serine and cysteine protease
family [Table 1]. When ECSAE was analyzed for its procoagulant effect
it decreased the clotting time of citrated human PRP from control
214.2s to 94.2s and promotes blood coagulation, at the maximum
concentration of 50μg after this dose it reached saturation (Figure 5).
ECSAE could significantly reduce the plasma recalcification time of
citrated human plasma suggested the procoagulant effect of ECSAE.
Clotting is the body’s normal response to prevent a person from
bleeding to death. However, blood clot formation can be dangerous if
it occurs within healthy blood vessels or if not degraded after due time.
Many diseases like heart attack, stroke and pulmonary embolism are
associated with inappropriate blood clot formation [19]. Although
procoagulant medicines made entirely of plants. Since proteases
have not yet been created, many plant extracts that contain them are
currently used extensively in traditional medicine to stop bleeding
and for wound healing. Several protein fractions and proteases from
plant latex have procoagulant properties that speed up clotting.
Serine and cysteine proteases from plant source exhibit procoagulant
action irrespective of the plant species and family. But the mechanism
involved in procoagulant action differs with the type of proteases [20].
In contrast, cysteine proteases exhibit specific effects on coagulation
factors. For example, ficin derived from Ficus carica shown to activate
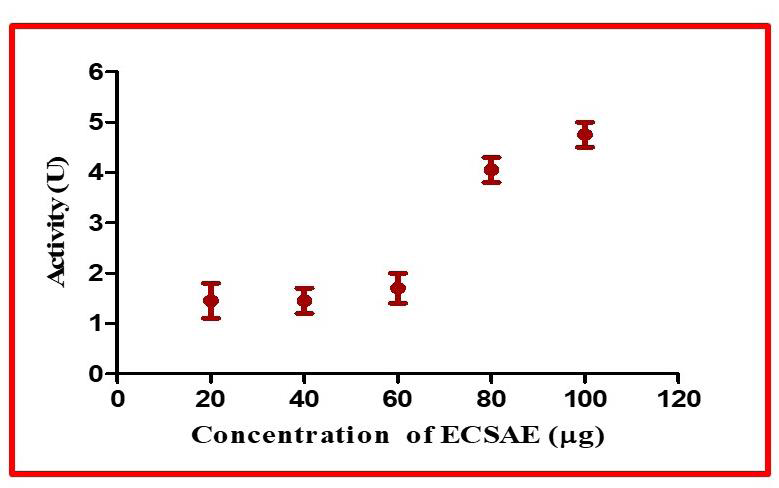
Figure 6:Fibrinolytic activity: Colorimetric assay. Washed plasma clot
was incubated with 20–100 μg of ECSAE for 2.30hr and then the OD was
measured at 660 nm.
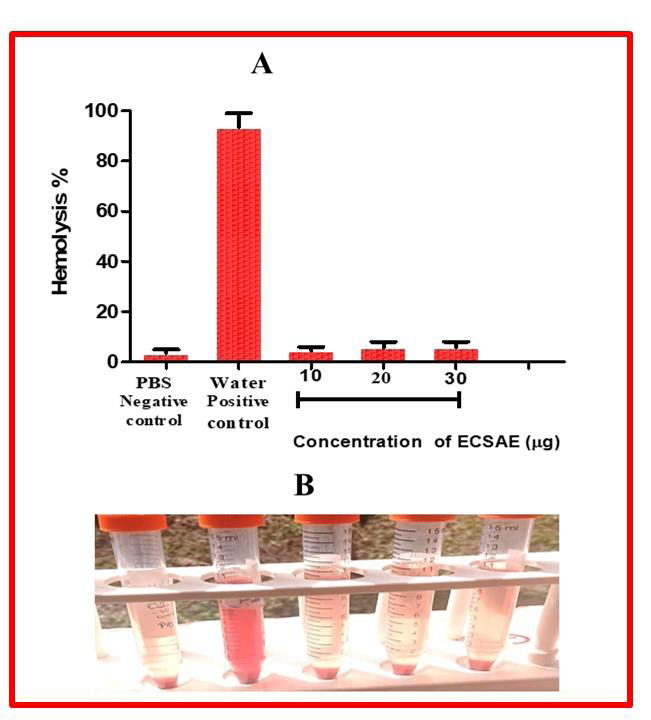
Figure 7:Direct Hemolytic assay. The different concentration of ECSAE
(10-30μg) was incubated independently for 1hr at 37° C with the 1 ml of
suspension made with packed human erythrocytes and phosphate buffered
saline (PBS) 1:9 v/v. The reaction was stopped by adding 9 ml of ice-cold
PBS and centrifuged at 1000g for 10min at 37°C. The amount of hemoglobin
released in the supernatant was measured at 540nm.
coagulation factor X, whereas Cysteine proteases from Asclepiadaceae
exhibit thrombin-like activity [21]. The fibrin clot dissolving ability of
ECSAE was carried out by colorimetric method. ECSAE hydrolyzed
the fibrin clot in a dose dependent manner and the specific activity was
found to be 1.1 to 4.5 units [Figure 6]. A Fibrinolytic agent induces
enzymatic activation of plasminogen to plasmin which cleaves the
fibrin molecules [22]. Consistent with the fibrinolytic effect of ECSAE
on fibrin, this supporting the procoagulant activity. ECSAE did not
hydrolyze RBC up to the concentration of 200 μg when compare to
the water positive control and PBS taken as a negative [Figure 7].
Conclusion
In conclusion, this study for the first time explores the antioxidant,
procoagulant, and fibrinolytic activities of protease(s) present in the
ECSAE. Thus, it could be better contender in the management of
thrombotic disorders.
Acknowledgment
The authors thank to Department of Biochemistry, Bangalore
University, Jnanabharathi Campus Bangalore- 560056.
Author and co-author contribution CD, PH and SH together planned and designed the research work. YR,KP and RR assisted in the laboratory work. CSK and HM reviewed the article.
Data availability The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author and co-author contribution CD, PH and SH together planned and designed the research work. YR,KP and RR assisted in the laboratory work. CSK and HM reviewed the article.
Data availability The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest:
The authors declare that we do not have a conflict of interest.Ethical clearance:
The research work does not involve any animals.